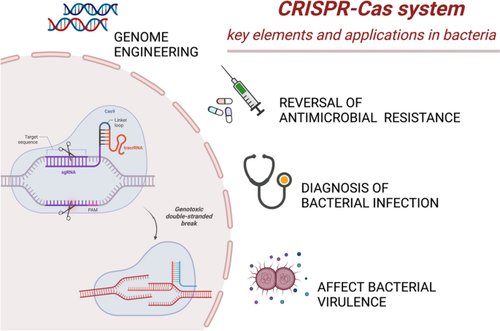
The CRISPR-Cas system has been harnessed as a revolutionary gene-editing tool due to its specificity and efficiency. It can introduce, remove, and modify genes in a wide range of organisms, not limited to its natural bacterial context.
The CRISPR-Cas system’s gene-editing abilities make it a promising tool in addressing the growing issue of antimicrobial resistance. It can specifically target and alter DNA sequences that encode antibiotic resistance genes, potentially reversing drug resistance in bacteria.
The CRISPR system can be programmed to target sequences specific to antibiotic resistance genes. Once identified, the CRISPR-associated proteins, such as Cas9, cut the DNA at these specific locations, disrupting the resistance gene and thus eliminating the resistance trait.
The CRISPR-Cas system can potentially combat antibiotic resistance by disrupting plasmid sequences — a typical method for antibiotic resistance spread. This disruption can limit horizontal gene transfer, modifying or eliminating resistance genes and potentially desensitizing bacteria to antibiotics.
The application of the CRISPR-Cas system in combating antimicrobial resistance is multifaceted. Here are the key points outlined in the text:
1. Genome Defense: CRISPR-Cas can defend against foreign genetic material such as those harboring antibiotic-resistant genes. This happens through interference with horizontal gene transfer, a predominant mechanism of drug resistance spread in bacteria.
2. Gene Editing: The CRISPR-Cas system can specifically target and cleave DNA sequences that encode antibiotic resistance genes. This precision allows for control and editing at the genetic level.
3. Prevention of Spread: It limits the spread of drug-resistant genes by recognizing and neutralizing foreign genetic elements that carry these genes. This prevents the propagation of antibiotic resistance.
4. Re-sensitizing Bacteria to Antibiotics: It can specifically target and eliminate plasmids that carry antibiotic resistance genes, therefore, re-sensitizing the bacteria to the antibiotics.
5. Consideration in Treatment Planning: Understanding the relationship between CRISPR-Cas system and antibiotic resistance could aid in developing new strategies for preventing and treating bacterial resistance.
The application of CRISPR-Cas9 could greatly align with Antibiotic stewardship programs following ways:
1. Rapid Diagnostics: CRISPR-Cas9 technology could be used to create highly sensitive and specific diagnostic tools, which could identify bacterial strains and their antibiotic resistance profiles rapidly and accurately. Earlier and more accurate diagnosis can help in the timely administration of the most effective antibiotics, which is one of the strategies of ASPs.
2. Treatment Personalization: CRISPR-Cas9 could also be used to personalize antibiotic treatments by editing or disabling genes involved in antibiotic resistance. The end result would be an engineered bacterium that is newly sensitive to a certain antibiotic.
3. Resistance Prevention: It has the potential to eliminate antibiotic resistance genes from pathogenic bacteria entirely. By precisely cutting out these genes, the spread of resistance within a bacterial population may be minimized.
4. Gene Drive Systems: CRISPR-Cas9 can be used to create gene drives that spread a desired genetic alteration through a bacterial population rapidly, potentially outpacing the development of antibiotic resistance.
5. Microbiome Engineering: CRISPR-Cas9 could be used to engineer the human microbiome, equipping our ‘good’ bacteria with the necessary tools to outcompete pathogenic, antibiotic-resistant ones.
In summary, the CRISPR-Cas9 system offers a promising method for tackling antibiotic resistance by directly altering resistance genes. This can complement antibiotic stewardship programs’ goals to use antibiotics judiciously, potentially encouraging better treatment outcomes and slowing the emergence of resistance. It opens a novel direction for innovative strategies in managing bacterial resistance.