In the battle against antibiotic resistance, short-cationic alpha-helical antimicrobial peptides (SCHAMPs) have emerged as promising contenders. These peptides are short in sequence, target bacteria selectively, and act swiftly by rupturing bacterial membranes. To unlock their full potential, understanding their mechanism of action is crucial. That’s where molecular dynamics (MD) simulations step in, offering detailed insights into how SCHAMPs interact with membranes at the atomic and meso-scale levels.
Key Insights:
- Binding and Insertion: SCHAMPs, including BP100, Decoralin, Neurokinin-1, and Temporin L, follow similar steps when interacting with membranes. They initially bind through electrostatic interactions, then flip on their axes, dehydrate, and insert their hydrophobic portions into the membrane core.
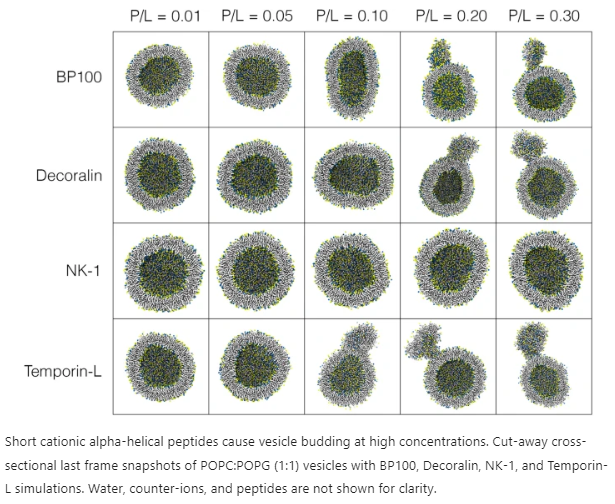
- Membrane Effects: At higher concentrations, SCHAMPs induce membrane budding and protrusions. This occurs as peptides continuously bind and flip into the membrane, leading to an imbalance in membrane volume and thickness. Ultimately, this imbalance triggers vesicle swelling and positive curvature, culminating in membrane budding.
- Wedge Model: The “wedge” model helps explain the differences in membrane outcomes among SCHAMPs. Peptides with a wider hydrophobic face and a smaller cluster of cationic residues (inverted wedge-shaped peptides) induce negative curvature, while those with a wider polar face of charged residues (wedge-shaped peptides) generate positive curvature.
- Amino Acid Composition: The number of large side-chain non-polar amino acids, such as leucine, phenylalanine, tyrosine, and tryptophan, plays a crucial role in SCHAMPs’ activity. Peptides with more hydrophobic residues exhibit higher membrane affinity and are more effective against bacteria.
Implications:
Understanding how SCHAMPs interact with membranes sheds light on their antimicrobial potential. By designing peptides with specific amino acid compositions and structural features, researchers can tailor their activity for enhanced effectiveness against antibiotic-resistant bacteria. However, it’s essential to balance potency with potential toxicity to human cells, especially for inverted wedge-shaped peptides like Temporin-L.
While MD simulations provide valuable insights, further research using improved methodologies and experimental validation is needed to fully harness the potential of SCHAMPs. Nonetheless, these findings represent a significant step forward in the fight against antibiotic resistance, offering new avenues for developing next-generation antimicrobial therapies.