💡 This review explores the intricate relationship between the host, oral medications, and gut microbiota, emphasizing the profound impact of microbial drug metabolism on drug absorption, bioavailability, efficacy, and toxicity. The bidirectional interaction between oral medications and gut microbes is discussed, highlighting the potential of drugs to shape microbial communities. Computational tools and machine learning approaches for predicting microbial drug metabolism are also explored, emphasizing the challenges and prospects in this evolving field.
📍 Microbial Influence on Drug Metabolism: Gut microbiota, housing over 3 million unique genes, significantly contributes to drug metabolism, impacting drug absorption, bioavailability, stability, efficacy, and toxicity. Microbial enzymatic reactions in the gut, distinct from hepatic metabolism, create a vast array of drug metabolites, influencing pharmacological effects.
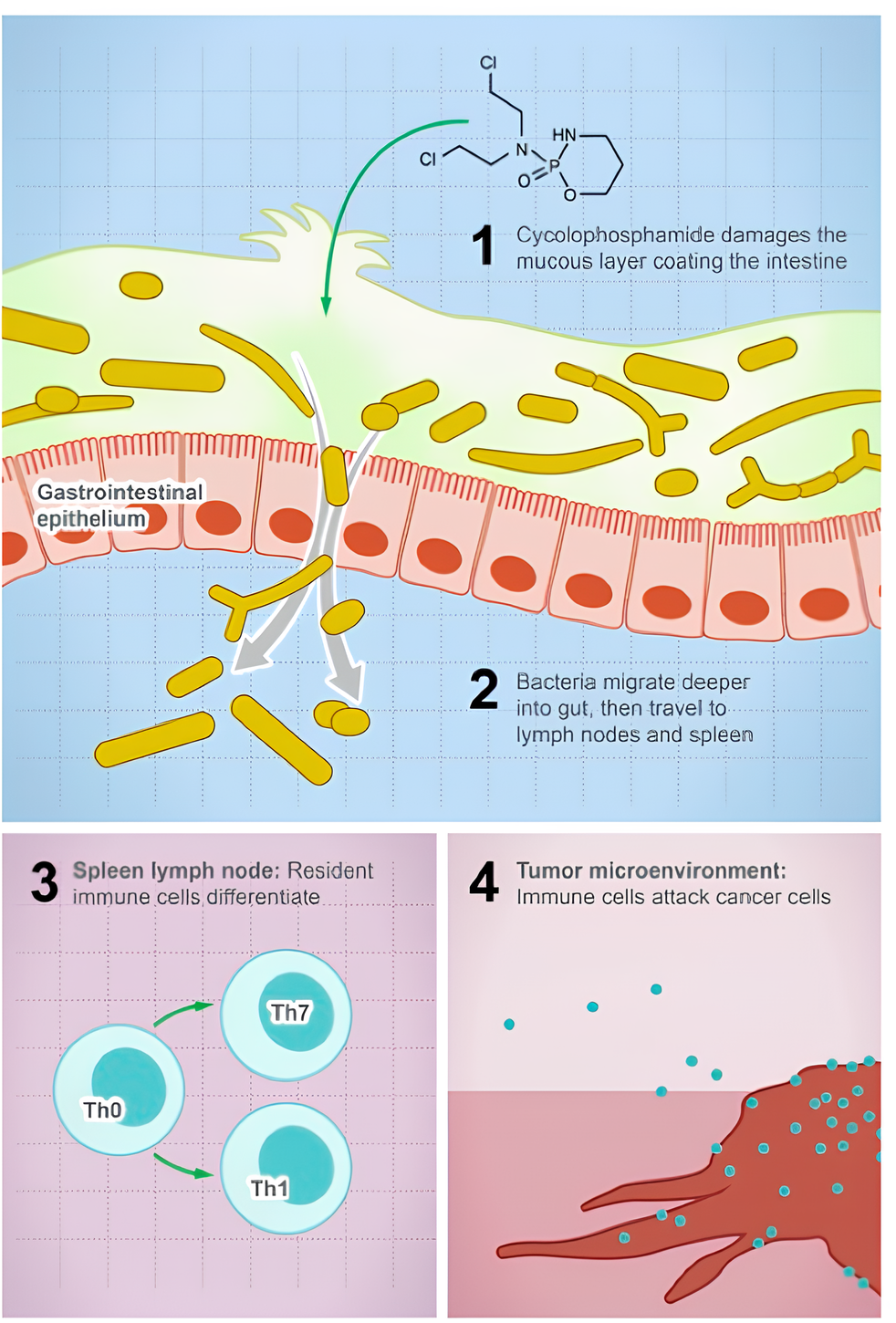
📍 Variability in Drug Response: Microbial deactivation of drugs, such as gemcitabine, can lead to chemotherapeutic resistance. Gut microbes play a crucial role in modulating the efficacy of anticancer drugs like cyclophosphamide, introducing variability in drug responses.
📍 Drug-Microbiota Bidirectional Interactions: Drugs can shape the composition, function, and gene expression of the gut microbial community.
Bidirectional interactions complicate predictions of drug-microbiota outcomes, challenging our ability to anticipate the consequences of drug administration.
📍 Functional Groups Susceptible to Microbial Metabolism:
Certain functional groups within oral medications are susceptible to microbial metabolism, influencing drug activation, inactivation, or toxicity.
Examples include ester, amide, nitro, and azo groups, with specific drugs like albiflorin, benzodiazepines, and antibacterial agents exhibiting susceptibility to microbial enzymatic degradation.
📍 Specific Examples of Microbial Drug Metabolism:
-Albiflorin, containing an ester group, undergoes hydrolysis by certain 𝘉𝘪𝘧𝘪𝘥𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢.
-Benzodiazepines are metabolized due to the presence of a nitro group.
-Antibacterial activities of various drugs, including prontosil and sulfasalazine, involve microbial azo reduction.
-Loperamide oxide’s antidiarrheal effect is mediated by gut bacteria cleaving N-oxide bonds.
-Levamisole incubation with human gut microbes results in thiazole ring-opened metabolites with anti-colon cancer activity.
📍 Microbial metabolism based on the intended use of xenobiotics, encompassing anticancer drugs, central nervous system (CNS) drugs, cardiovascular drugs, steroids, supplements, and natural products.
📍 Anticancer Drugs:
📌 Microbial Resistance and Metabolism:
Inconsistent responses and resistance to anticancer therapeutics are linked to the gut microbiome microenvironment.
𝘌. 𝘤𝘰𝘭𝘪 𝘢𝘯𝘥 𝘓𝘪𝘴𝘵𝘦𝘳𝘪𝘢 𝘸𝘦𝘭𝘴𝘩𝘪𝘮𝘦𝘳𝘪 have been associated with resistance to chemotherapeutics.
𝘌. 𝘤𝘰𝘭𝘪 degrades anticancer drugs, including tretazicar, gemcitabine, and doxorubicin, impacting drug efficacy.
📌 Immune Checkpoint Inhibitors (ICIs) and Gut Microbes:
Antibiotics with anti-PD-1 ICIs reduce progression-free survival (PFS) and overall survival (OS), highlighting the impact of gut microbes.
Specific microbial species, such as 𝘈𝘭𝘪𝘴𝘵𝘪𝘱𝘦𝘴 𝘪𝘯𝘥𝘪𝘴𝘵𝘪𝘯𝘤𝘵𝘶𝘴, 𝘈𝘬𝘬𝘦𝘳𝘮𝘢𝘯𝘴𝘪𝘢 𝘮𝘶𝘤𝘪𝘯𝘪𝘱𝘩𝘪𝘭𝘢, 𝘢𝘯𝘥 𝘌𝘯𝘵𝘦𝘳𝘰𝘤𝘰𝘤𝘤𝘶𝘴 𝘩𝘪𝘳𝘢𝘦, restore ICI efficacy.
Gut microbial signatures serve as potential biomarkers for predicting ICIs’ treatment response.
📌 Microbial Impact on Ipilimumab Treatment:
Gut microbial variations predict poor ipilimumab treatment response in metastatic melanoma patients.
Bacterial operational taxonomic units (OTUs) act as potential biomarkers for ipilimumab-colitis free patients.
📍 Central Nervous System Drugs:
📌 Metabolism of Anti-Depressants and Anxiolytics:
𝘉𝘪𝘧𝘪𝘥𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 strains metabolize the anti-depression drug albiflorin.
Microbes, including 𝘌. 𝘤𝘰𝘭𝘪, metabolize clonazepam, nitrazepam, and flunitrazepam through nitro-reduction.
📌 Gut Microbes and Levodopa Response:
Gut microbes, including 𝘌𝘯𝘵𝘦𝘳𝘰𝘤𝘰𝘤𝘤𝘶𝘴 𝘧𝘢𝘦𝘤𝘢𝘭𝘪𝘴 𝘢𝘯𝘥 𝘌𝘨𝘨𝘦𝘳𝘵𝘩𝘦𝘭𝘢 𝘭𝘦𝘯𝘵𝘢, metabolize levodopa, affecting treatment response.
Levodopa metabolites produced by 𝘊𝘭𝘰𝘴𝘵𝘳𝘪𝘥𝘪𝘶𝘮 𝘴𝘱𝘰𝘳𝘰𝘨𝘦𝘯𝘦𝘴 exhibit variable deamination, influencing therapeutic outcomes.
📍 Cardiovascular Drugs:
Digoxin is reduced by 𝘌𝘨𝘨𝘦𝘳𝘵𝘩𝘦𝘭𝘢 𝘭𝘦𝘯𝘵𝘢 in the gut.
Gut microbes, affected by antibiotics, influence the response to quinapril and amlodipine in hypertension patients.
Lovastatin treatment response is altered due to microbial metabolism.
📍 Steroids and Corticosteroids:
Microbial enzymes in 𝘗𝘳𝘰𝘵𝘦𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 𝘢𝘯𝘥 𝘈𝘤𝘵𝘪𝘯𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 degrade steroids.
Gut microbes, including 𝘊𝘭𝘰𝘴𝘵𝘳𝘪𝘥𝘪𝘶𝘮 𝘴𝘤𝘪𝘯𝘥𝘦𝘯𝘴, impact the metabolism of external corticosteroids.
Estrogen is reactivated by gut microbes through B-glucuronidase activity.
📍 Miscellaneous Xenobiotics and Natural Substances:
Gut microbes affect vitamin D levels, with conflicting results on vitamin D supplementation.
Microbial impact on natural substances like chlorogenic acid involves cinnamoyl esterase activity.
📍 Computational Prediction of Microbial Drug Metabolism:
Tools like MASI and MagMD provide databases for experimentally determined drug-microbe interactions. Machine learning models, including Drug Bug and others, predict drug degradation by gut microbes, considering microbial diversity variations. Meta-analysis studies reveal associations between specific drugs and microbial species, emphasizing the bidirectional nature of drug-microbiome interactions.
This comprehensive exploration of microbial metabolism of xenobiotics underscores the need for personalized approaches in drug development and treatment strategies, considering the intricate interplay between drugs, gut microbes, and host responses. Advances in computational prediction tools hold promise for enhancing our understanding of pharmacomicrobiomics.
Link to the article : http://tinyurl.com/3c3smutu