𝘔𝘺𝘤𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘵𝘶𝘣𝘦𝘳𝘤𝘶𝘭𝘰𝘴𝘪𝘴 infection is associated with dysregulation of the immune system, leading to alterations in the gut microbiome. This study synthesizes key scientific findings from various studies investigating the impact of Tuberculosis (TB) on the gut microbiome and its potential immunological consequences.
📍 General Gut Microbiome Alterations in TB Patients
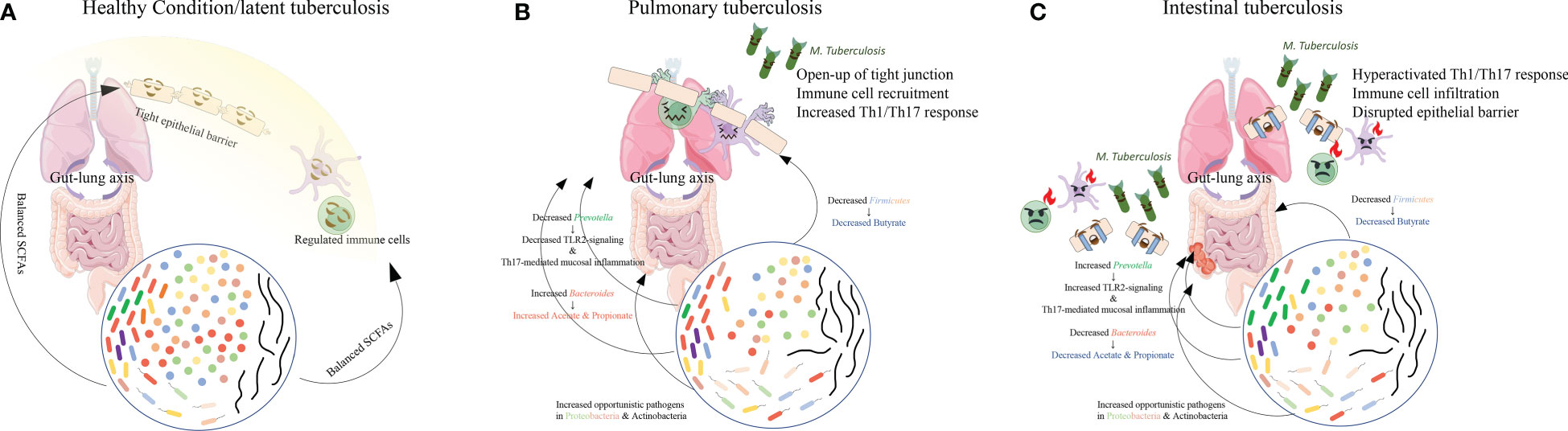
Mouse models challenged with M. tuberculosis exhibited dysbiosis resembling that observed in TB patients. Dysbiosis was characterized by a rapid reduction in alpha-diversity followed by partial recovery, suggesting a dynamic interplay between the microbiota and immune system.
📍 Taxonomic Dysregulation
📌 Phylum 𝘍𝘪𝘳𝘮𝘪𝘤𝘶𝘵𝘦𝘴
Significant reduction in 𝘍𝘪𝘳𝘮𝘪𝘤𝘶𝘵𝘦𝘴, particularly within 𝘊𝘭𝘰𝘴𝘵𝘳𝘪𝘥𝘪𝘢𝘭𝘦𝘴 𝘢𝘯𝘥 𝘝𝘦𝘪𝘭𝘭𝘰𝘯𝘦𝘭𝘭𝘢𝘭𝘦𝘴.
Families 𝘓𝘢𝘤𝘩𝘯𝘰𝘴𝘱𝘪𝘳𝘢𝘤𝘦𝘢𝘦, 𝘙𝘶𝘮𝘪𝘯𝘰𝘤𝘰𝘤𝘤𝘢𝘤𝘦𝘢𝘦, 𝘢𝘯𝘥 𝘊𝘭𝘰𝘴𝘵𝘳𝘪𝘥𝘪𝘢𝘤𝘦𝘢𝘦, along with genera like 𝘍𝘢𝘦𝘤𝘢𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮, 𝘙𝘶𝘮𝘪𝘯𝘰𝘤𝘰𝘤𝘤𝘶𝘴, 𝘢𝘯𝘥 𝘉𝘭𝘢𝘶𝘵𝘪𝘢,
were consistently decreased.
Reduced levels of obligate anaerobic and butyrate-producing bacteria within 𝘍𝘪𝘳𝘮𝘪𝘤𝘶𝘵𝘦𝘴.
Immunological Implications
Decreased butyrate levels may lead to elevated pro-inflammatory responses, reduced antimicrobial activity, and impaired epithelial barrier function.
Butyrate’s role in inducing antimicrobial peptides and enhancing the clearance of 𝘔𝘺𝘤𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘵𝘶𝘣𝘦𝘳𝘤𝘶𝘭𝘰𝘴𝘪𝘴 is crucial.
📌 Phylum 𝘉𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘵𝘦𝘴
Conflicting findings, but predominant observations included increased 𝘉𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴 𝘢𝘯𝘥 𝘗𝘢𝘳𝘢𝘣𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴 and decreased 𝘗𝘳𝘦𝘷𝘰𝘵𝘦𝘭𝘭𝘢.
Acetate and propionate-producing bacteria, 𝘉𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴 𝘢𝘯𝘥 𝘗𝘢𝘳𝘢𝘣𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴, were elevated.
Immunological Implications
Acetate and propionate may enhance antimicrobial peptides and contribute to epithelial barrier repair.
𝘗𝘳𝘦𝘷𝘰𝘵𝘦𝘭𝘭𝘢 reduction may exert an anti-inflammatory effect, potentially balancing the pro-inflammatory response.
📌 𝘗𝘳𝘰𝘵𝘦𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 𝘢𝘯𝘥 𝘈𝘤𝘵𝘪𝘯𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢
𝘗𝘳𝘰𝘵𝘦𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 increased, with genera like 𝘗𝘴𝘦𝘶𝘥𝘰𝘮𝘰𝘯𝘢𝘴 𝘢𝘯𝘥 𝘌𝘴𝘤𝘩𝘦𝘳𝘪𝘤𝘩𝘪𝘢 elevated.
Conflicting trends for , 𝘈𝘤𝘵𝘪𝘯𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 but 𝘈𝘤𝘵𝘪𝘯𝘰𝘮𝘺𝘤𝘦𝘴 was reported to increase.
Immunological Implications
Opportunistic pathogens associated with 𝘗𝘳𝘰𝘵𝘦𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 𝘢𝘯𝘥 𝘈𝘤𝘵𝘪𝘯𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢 may exploit a disrupted gut environment, exacerbating immune dysregulation.
📍 Microbiome-Immune Crosstalk in 𝘔. 𝘵𝘶𝘣𝘦𝘳𝘤𝘶𝘭𝘰𝘴𝘪𝘴 Infection
The gut-lung axis plays a crucial role in shaping the immune response during 𝘔. 𝘵𝘶𝘣𝘦𝘳𝘤𝘶𝘭𝘰𝘴𝘪𝘴 infection.
Short-chain fatty acids (SCFAs), especially butyrate, influence immune modulation and epithelial function.
Dysbiosis in TB patients may lead to altered SCFA composition, impacting the lung microbiome, epithelial barrier, and systemic cytokine levels.
📍 Immunological Consequences in PTB and ITB Patients
PTB patients exhibited dysbiosis characterized by reduced butyrate and increased acetate and propionate.
ITB patients showed further depletion of SCFAs, potentially leading to excessive immune responses, increased inflammation, and impaired antimicrobial defenses.
📍 TB treatment-induced dysbiosis raises concerns about treatment efficacy, emphasizing the need for strategies to maintain a balanced gut microbiome.
Probiotics and postbiotics, such as 𝘉𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴 𝘧𝘳𝘢𝘨𝘪𝘭𝘪𝘴 and indole propionic acid, show promise as supplements during TB treatment. Personalized approaches considering the concentration and type of microbial interventions are crucial.
Link to the article : http://tinyurl.com/bdeekcaz