💡 The global surge in age-related cardiovascular diseases has prompted extensive research into novel therapeutic avenues. This review delves into the intricate relationship between gut microbiota composition and age-related cardiovascular diseases, emphasizing key metabolites like trimethylamine N-oxide (TMAO), bile acids (BAs), and short-chain fatty acids (SCFAs). The study explores molecular pathways affected by gut microbiota dysbiosis and suggests the gut microbiota as a promising therapeutic target for mitigating cardiovascular diseases.
📍 Atherosclerosis:
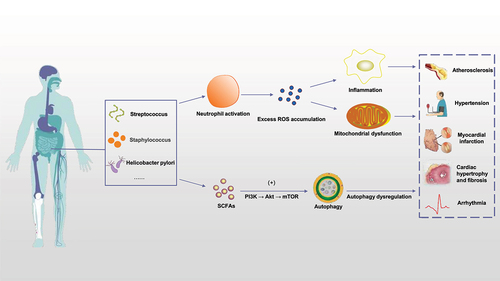
Atherosclerotic plaques harbor diverse bacterial DNA, indicating a microbial environment.
Patients with atherosclerotic cardiovascular disease exhibit altered gut microbiota, particularly higher levels of 𝘚𝘵𝘳𝘦𝘱𝘵𝘰𝘤𝘰𝘤𝘤𝘶𝘴 𝘢𝘯𝘥 𝘌𝘯𝘵𝘦𝘳𝘰𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘢𝘤𝘦𝘢𝘦.
Gut microbiota dysbiosis contributes to atherosclerosis development through oxidative stress and inflammation.
Microbial metabolites like TMAO promote atherosclerosis by inducing endothelial dysfunction and inflammation.
📍 Hypertension:
Gut-brain axis dysfunction is implicated in hypertension, involving bidirectional communication between gut microbiota and the brain.
Neuroinflammation triggered by gut-brain axis dysfunction elevates inflammatory mediators, impacting blood pressure regulation.
Long-term high-salt diets increase TMAO levels, promoting neuroinflammation and oxidative stress, contributing to hypertension.
📍 Myocardial Infarction and Heart Failure:
Patients with myocardial infarction and heart failure experience increased intestinal permeability and bloodstream infections.
𝘓𝘢𝘤𝘵𝘰𝘣𝘢𝘤𝘪𝘭𝘭𝘶𝘴, 𝘉𝘢𝘤𝘵𝘦𝘳𝘰𝘪𝘥𝘦𝘴, 𝘢𝘯𝘥 𝘚𝘵𝘳𝘦𝘱𝘵𝘰𝘤𝘰𝘤𝘤𝘶𝘴 from the gut are detected in blood microbiomes of patients with myocardial infarction.
Intestinal bacteria and LPS in systemic circulation trigger monocyte recruitment, systemic inflammation, and cardiovascular events.
SCFAs, particularly butyrate, contribute to cardiac repair and improved prognosis after myocardial infarction.
📍 Myocardial Hypertrophy and Fibrosis:
Probiotics, prebiotics, and biotin administration improves gut microbiota composition, reducing cardiac hypertrophy induced by hypobaric hypoxia.
LPS induces cardiac oxidative stress, myocardial and perivascular fibrosis, linking gut microbiota to pathological cardiac remodeling.
Elevated TMAO levels are associated with heart failure, left ventricular dysfunction, and increased susceptibility to ventricular arrhythmias.
📍 Arrhythmia:
𝘍𝘢𝘦𝘤𝘢𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘢𝘯𝘥 𝘖𝘴𝘤𝘪𝘭𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳 abundance decreases in patients with atrial fibrillation, while 𝘙𝘶𝘮𝘪𝘯𝘰𝘤𝘰𝘤𝘤𝘶𝘴, 𝘚𝘵𝘳𝘦𝘱𝘵𝘰𝘤𝘰𝘤𝘤𝘶𝘴, 𝘢𝘯𝘥 𝘌𝘯𝘵𝘦𝘳𝘰𝘤𝘰𝘤𝘤𝘶𝘴 increase.
SCFAs attenuate NLRP3 inflammasome activation, reducing the occurrence of atrial fibrillation.
High TMAO levels promote atrial fibrillation by inducing left atrial inflammation and fibrosis.
PAGln, a gut microbiota-derived metabolite, increases oxidative stress and atrial cell apoptosis, promoting atrial fibrillation.
📍 Gut Microbiome as a Potential Therapeutic Direction:
Fecal microbiota transplantation (FMT) shows promise in restoring microbial balance and improving insulin sensitivity in patients with cardiometabolic syndrome.
Specific inhibition of FXR in the intestine could reduce TMAO production without affecting cholesterol metabolism.
Maintaining a healthy gut flora through dietary optimization, prebiotics, and probiotics emerges as crucial for cardiovascular health.
Link to the article : http://tinyurl.com/yc8z2yhj