Pancreatitis, characterized by inflammation of the pancreas, has been associated with alterations in gut microbiota composition, although the specific changes and underlying mechanisms remain unclear. Understanding the interplay between pancreatitis and gut microbiota, metabolites, and inflammatory cytokines is crucial for elucidating the pathogenesis and potential treatment strategies.
This study aimed to investigate the causal relationships between pancreatitis and these factors using two-sample Mendelian randomization (MR) analysis. By examining genetic associations, this research provides insights into the complex interactions shaping the gut–pancreas axis and identifies potential mediators involved in pancreatitis progression.
Key Scientific Findings:
Genetic Associations with Pancreatitis Types:
- Chronic pancreatitis (CP) showed genetic associations with decreased serum citrate levels and alterations in amino acid metabolism.
- Alcohol-induced chronic pancreatitis (AICP) exhibited genetic associations with elevated serum pyruvate levels and disrupted amino acid metabolism, including low levels of valine, tyrosine, and leucine.
- Acute pancreatitis (AP) demonstrated genetic associations with lipids, including unsaturated fatty acids, cholesterol, and high-density lipoprotein (HDL) levels.
Role of Metabolites in Pancreatitis Pathogenesis:
- Pyruvate, a key metabolite linking glycolysis and the tricarboxylic acid (TCA) cycle, was genetically associated with AICP, indicating its potential role in alcohol-induced pancreatic damage.
- Reduced serum citrate and tyrosine concentrations in CP patients suggest disturbances in glucose and amino acid metabolism, contributing to pancreatic inflammation and fibrosis.
Inflammatory Cytokine Profile in Pancreatitis:
- Genetic associations were found between CP and elevated levels of IL-4, IL-10, IL-12, and stem cell growth factor (SCGF-β), indicating immune dysregulation and fibrotic processes.
- AP showed genetic associations with increased IL-6 levels, suggesting its role as a marker for severity and prognosis, while low IL-1β levels were associated with alcohol-induced acute pancreatitis (AIAP).
Gut Microbiota Alterations:
- CP was genetically associated with decreased abundance of Lentisphaerae and Ruminococcus torques group, while Eubacterium brachy and Lachnospiraceae UCG001 were increased, suggesting microbial dysbiosis.
- AICP exhibited decreased abundance of Candidatus soleaferrea, Eubacterium fissicatena, and Ruminococcaceae UCG009, with increased levels of Erysipelotrichaceae and Eggerthella.
- AP was associated with increased abundance of Bacteroides, Proteobacteria, Odoribacter, and Butyricicoccus, indicating disturbances in gut microbiota composition.
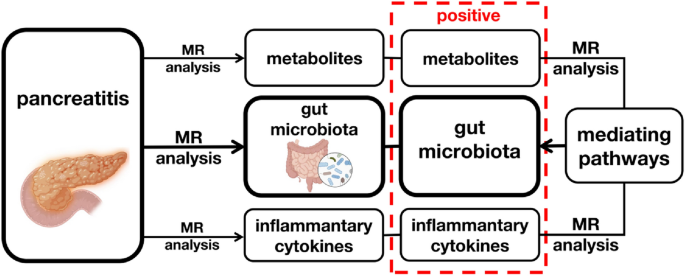
Mediating Roles of Metabolites and Cytokines:
- Pyruvate and free cholesterol in very large HDL were identified as potential mediators between pancreatitis and specific gut microbiota alterations.
- Inflammatory cytokines such as IL-4, IL-10, IL-12, and SCGF-β may mediate the effects of pancreatitis on gut microbiota composition, indicating intricate immune-microbiota interactions.
This study provides comprehensive insights into the genetic associations and potential mediating pathways underlying the complex interplay between pancreatitis, gut microbiota, metabolites, and inflammatory cytokines. Understanding these interactions sheds light on the pathogenesis of pancreatitis and offers new avenues for targeted therapies, emphasizing the importance of restoring gut microbiota balance and modulating metabolic and immune responses to mitigate pancreatitis progression. Further research is warranted to elucidate the underlying mechanisms and develop personalized interventions for pancreatitis management.
Link to the article : https://tinyurl.com/3hsu723n