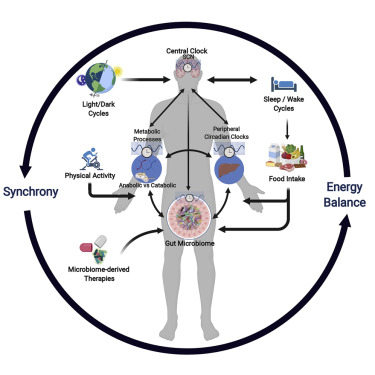
The interplay between circadian rhythms and the gut microbiome is a complex and dynamic relationship that significantly influences metabolic processes and overall health. Circadian rhythms are recurring physiological cycles that follow a 24-hour pattern and are influenced by external stimuli such as light and darkness. These rhythms are essential for maintaining homeostasis and synchronizing numerous physiological functions, such as sleep-wake cycles, hormone release, and metabolic activity. The gut microbiome is a varied population of bacteria that live in the gastrointestinal tract and play an important role in digestion, nutritional absorption, and immune system regulation. The complex interaction of these two systems has significant implications for metabolic conditions such as obesity, diabetes, and nonalcoholic fatty liver disease.
This relationship is based on bidirectional communication between the central circadian clock, This link is based on bidirectional communication between the central circadian clock, which is located in the brain’s suprachiasmatic nucleus, and peripheral clocks found in numerous organs such as the liver, intestine, and adipose tissue. These clocks coordinate the timing of physiological activities to match environmental cues, optimizing energy usage and metabolic efficiency. The gut microbiome is impacted by circadian rhythms and helps to regulate the host’s internal clock.
Recent metagenomic studies have revealed innate circadian rhythms in gut bacteria, with up to 20% of these microorganisms exhibiting diurnal changes in abundance and activity. These oscillations point to a synchronized rhythm with host physiology, highlighting the coordinated nature of the circadian-microbiome interplay. Notably, investigations on human stool samples have shown that 𝘌𝘯𝘵𝘦𝘳𝘰𝘣𝘢𝘤𝘵𝘦𝘳 𝘢𝘦𝘳𝘰𝘨𝘦𝘯𝘦𝘴, a common gut bacterium, responds to the circadian hormone melatonin, implying a direct relationship between the host’s circadian signaling and microbial behavior.
Microbial influences on circadian rhythms are emphasized further by the significance of signaling chemicals produced by specific gut bacteria, such as butyrate. Butyrate, a short-chain fatty acid, is an important mediator in resetting hepatic circadian and intestine peripheral clock gene expression. Furthermore, feeding patterns cause rhythmic oscillations in microbiotal bacterial composition, which have a significant impact on the host’s circadian gene expression. The gut epithelium, equipped with toll-like receptors, has a rhythmic pattern in sensing microbiotal metabolites, which contributes to the regulation of circadian gene expression in the liver and intestines.
Bile acid signaling emerges as a key participant in the complex interplay between circadian rhythms and the gut microbiome. Microbes in the intestinal lumen help deconjugate bile salts into unconjugated bile acids. These unconjugated bile acids function as signaling molecules, regulating the amplitude and timing of circadian gene expression in the ileum, colon, and liver. The link between bile acid metabolism and circadian rhythm emphasizes the host’s symbiotic relationship with its microbial residents.
Studies with germ-free mice, which lack intestinal microbiota, provide light on the bidirectional relationship between circadian rhythms and the gut microbiome. Mice lacking a microbiome exhibit altered circadian gene expression in response to dietary perturbations, indicating the microbiota’s function in influencing the host’s circadian response, particularly to dietary changes. On the other hand, changing circadian rhythms in healthy mice by changes in the light-dark cycle and genetic mutations affects the gut microbiota composition, resulting in dysbiosis defined by an imbalance of proinflammatory and anti-inflammatory bacterial species.
Dietary parameters, such as mealtime and diet composition, play an important role in the circadian-microbiome interplay. High-fat diets have been demonstrated to reduce cerebral and hepatic circadian clock gene expression, slow gut microbiota circadian oscillations, and change microbial diversity. Time-restricted feeding, a type of intermittent fasting with daily fasting periods, has an impact on gut bacteria composition and diversity. In a multicenter study, adults who practiced time-restricted meals showed increased gut microbial diversity, emphasizing the importance of meal timing on the microbiome.
Understanding this complex dynamic could result in significant therapeutic implications. Circadian rhythms and the gut microbiome are interesting targets for therapies to prevent and treat metabolic disorders. The restoration of normal circadian rhythms through measures such as light exposure, timed eating regimens, and reduced shift work shows promise in maintaining metabolic balance. Microbial therapies such as dietary changes, prebiotics, probiotics, and fecal microbiota transplants offer opportunities to control the gut microbiome and positively impact host metabolism.
Further Reading :
Timing the Microbes: The Circadian Rhythm of the Gut Microbiome : http://tinyurl.com/5f2aynpk
Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health : http://tinyurl.com/2zkbkdm5
Circadian Rhythms, the Gut Microbiome, and Metabolic Disorders : http://tinyurl.com/2en3tzuu
Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition : http://tinyurl.com/yc4r2zr7