The complex interactions between host-produced hormones and human-associated bacteria have been brought to light by recent studies, but the underlying processes and physiological effects are still mostly unknown.
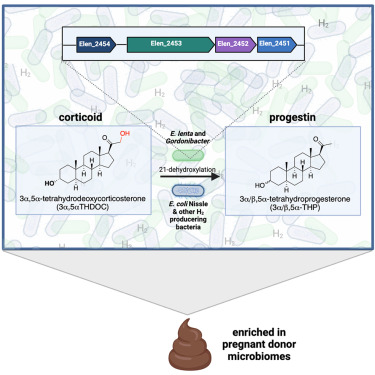
This study focuses on the gut bacteria Gordonibacter pamelaeae and Eggerthella lenta, which use a process known as 21-dehydroxylation to change biliary corticoids into progestins. This conversion yields sex hormones and neurosteroids from a class of steroids important in immunological and metabolic control.
The study identifies the genetic underpinnings of this conversion and highlights the critical role that gut microbe-produced hydrogen gas plays in promoting the 21-dehydroxylation process.
Pregnancy-related elevations in some progestin levels raise the possibility that bacterial steroid metabolism may have significant effects on host physiology, especially for the health of women. This work offers fundamental understanding of how bacteria regulate steroid metabolism.
METHODS
- Comparative Genomics: Comparative and functional genomics were used to identify the gene cluster involved in 21-dehydroxylation.
- Homologous and Heterologous Expression: Techniques were employed to confirm the function of the identified gene cluster.
- Co-culture Experiments: E. lenta was co-cultured with E. coli mutants to study the role of H2 in promoting 21-dehydroxylation.
- Gas Atmosphere Experiments: E. lenta monocultures were maintained under different gas atmospheres (H2 vs. N2) to evaluate the impact on 21-dehydroxylation.
- Fecal Analysis: Fecal samples from pregnant and non-pregnant humans and mice were analyzed to assess progestin levels and gene cluster abundance.
KEY FINDINGS
- Biliary corticoids are converted into progestins by the human gut bacteria Gordonibacter pamelaeae and Eggerthella lenta by 21-dehydroxylation, which also converts immuno- and metabo-regulatory steroids into sex hormones and neurosteroids.
- Hydrogen gas produced by gut commensals significantly promotes the 21-dehydroxylation process. 21-dehydroxylation is enhanced when E. lenta is cultured in combination with H2-producing E. coli or when E. lenta monocultures are kept in an H2 environment.
- Using comparative and functional genomics, a bacterial gene cluster (Elen_2451–2454) responsible for 21-dehydroxylation was found, suggesting the involvement of iron-sulfur binding domain proteins and molybdenum-dependent oxidoreductase.
- Progestin production by bacteria affects host physiology, notably in women’s health, as evidenced by the significantly greater levels of progestin during pregnancy, especially allopregnanolone (brexanolone) and the gene cluster abundance.
- H2 may donate electrons to generate the ferredoxin cofactor needed for 21-dehydroxylation, or it may directly supply reducing equivalents. Further research is needed to clarify the precise mechanism in question.
Link to the study : https://tinyurl.com/mrxfz3pz