💡 This study investigates the intricate interplay between dietary factors, gut microbiota, and subtypes of Irritable Bowel Syndrome (IBS). Using data from the ZOE PREDICT 1 study
📌 They identified associations between dietary habits and microbiome composition in individuals with predominant bowel habits of diarrhea (IBS-D) and constipation (IBS-C).
📌 Specific microbiota variations and their impact on diet-microbiota interactions are revealed, offering insights for subtype-specific dietary interventions. The study underscores the potential role of the gut microbiome in shaping the IBS symptomology and paves the way for microbiome-informed personalized dietary approaches in IBS management.
📍 Methods:
Study Design: Utilized data from the ZOE PREDICT 1 study, focusing on 969 participants aged 18-65, with dietary information and stool metagenome data. Classified individuals into IBS subtypes (IBS-D and IBS-C) based on Rome III criteria.
Data Analysis: Employed multivariable-adjusted linear regression to identify microbiota variations in IBS subtypes. Investigated diet-microbiota interactions in relation to IBS subtypes.
📍 Key Findings:
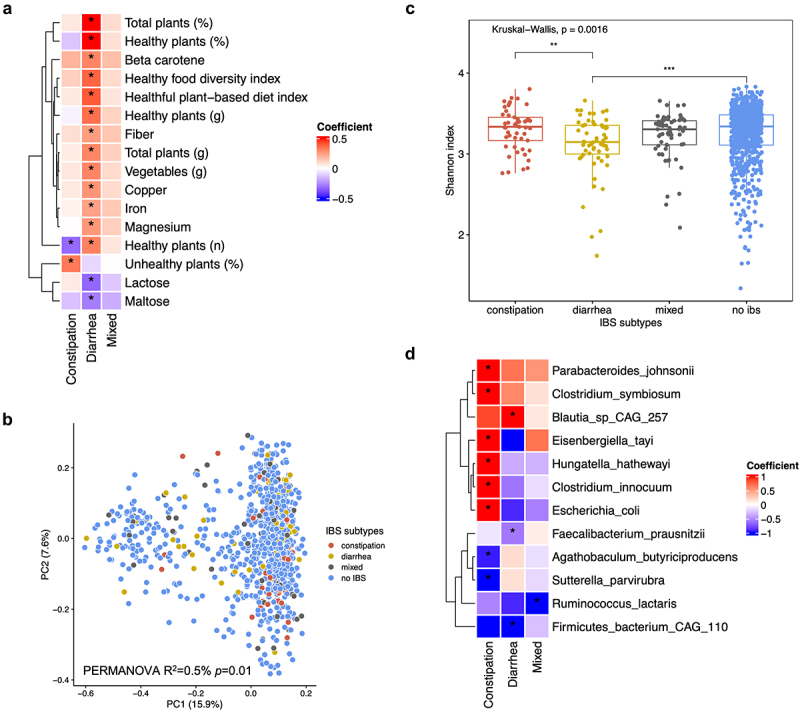
Dietary Associations: IBS-D participants consumed healthier plant-based foods, fiber, magnesium, and iron, while IBS-C participants tended to consume less healthy plant-based foods, maltose, and lactose.
Microbiota Variations: IBS-D was associated with a loss of butyrate-producing anaerobic bacteria like 𝘍𝘢𝘦𝘤𝘢𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘱𝘳𝘢𝘶𝘴𝘯𝘪𝘵𝘻𝘪𝘪, leading to decreased microbial diversity. IBS-C exhibited slight increases in pro-inflammatory taxa, including Escherichia coli.
Diet-Microbiota Interactions: Stronger associations between fiber and iron intake and IBS-D were observed in participants with higher 𝘍𝘢𝘦𝘤𝘢𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘱𝘳𝘢𝘶𝘴𝘯𝘪𝘵𝘻𝘪𝘪 relative abundance. IBS-D is linked to the consumption of healthier foods, while IBS-C is associated with microbiota changes potentially driving inflammation.
Butyrate and Inflammation: Loss of butyrate-producing anaerobic bacteria in IBS-D may contribute to abdominal pain, suggesting potential treatments with butyrate supplementation. Increased pro-inflammatory taxa in IBS-C raise concerns about colorectal cancer and gut-brain axis implications.
Diet-Microbiota Interactions: Associations between fiber and iron intake and IBS-D are strengthened by higher 𝘍𝘢𝘦𝘤𝘢𝘭𝘪𝘣𝘢𝘤𝘵𝘦𝘳𝘪𝘶𝘮 𝘱𝘳𝘢𝘶𝘴𝘯𝘪𝘵𝘻𝘪𝘪 abundance. Implications for glucuronidase and potential interference with dietary benefits in IBS-D.
📌 This research uncovers subtype-specific dietary habits, gut microbiota composition, and diet-microbiota interactions in IBS. The findings offer insights into potential biomarkers and microbiome-informed, personalized dietary interventions for IBS treatment. Future longitudinal studies and randomized controlled feeding trials are warranted to validate and further explore these associations.
Link to the article : https://tinyurl.com/39my5wp2