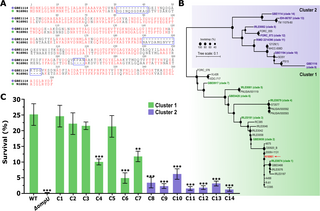
Antimicrobial resistance is a complex phenomenon fueling the microbial “arms race” to combat emerging and re-emerging pathogens. Gram-negative pathogens pose a major obstacle towards tackling this as the presence of their highly selective outer membrane (OM) critically facilitate adaptations for AMR. Porins represent one of the major proteins present in the OM and are often directly implicated in AMR. OmpU, a homotrimer porin that is distributed throughout up to 60% of the OM of V. cholerae. OMPs modulate transport of small molecules, with selectivity allowing for the intake of essential nutrients, or preventing internalization of antimicrobials. Furthermore, alteration in levels of OMP expression, mutations in the porin constriction region, or expression of alternative porins can confer AMR. Authors in this study, developed a framework that exploits genetic diversity from environmental bacterial populations to decode emergent phenotypes like AMR. They identified and characterized functional domains in OmpU unique to variants conferring AMR-associated phenotypes. Specifically, four conserved domains that are linked with resistance to bile and host-derived antimicrobial peptides. The findings of this study, highlight the astonishing nuances of OmpU and its multifaceted role in the emergence and pathogenesis of V. cholerae.
Link to the article: bit.ly/44ZyecL
Published On: /05/2023