Postoperative atrial fibrillation (POAF) is a prevalent complication following coronary artery bypass grafting (CABG) surgery. The gut microbiota and its metabolites have been proposed to play a role in the progression and development of atrial fibrillation (AF) through their interaction with multiple functional pathways via the gut-heart axis.
This study aimed to identify alterations in the fecal microbiome and plasma metabolome that distinguish POAF from non-POAF patients undergoing isolated CABG. Using 16S rRNA sequencing and targeted/untargeted metabolomic profiling, the study investigated the gut microbiota metabolism biomarkers and related risk models associated with POAF.
Methods
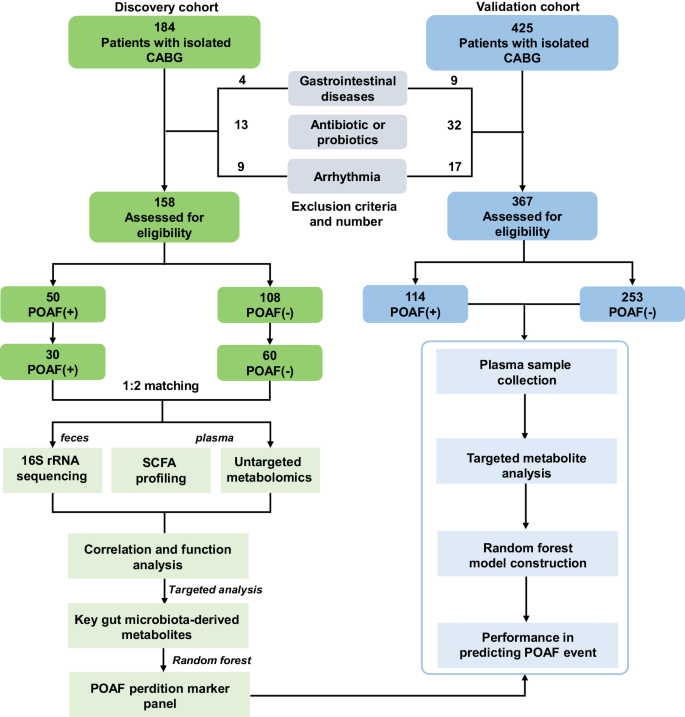
- 16S rRNA Sequencing: This technique was employed to analyze the fecal microbiome of POAF and non-POAF patients, allowing for the identification and comparison of bacterial taxa.
- Targeted/Untargeted Metabolomic Profiling: Plasma samples were analyzed to identify and quantify metabolites, focusing on short-chain fatty acids (SCFAs) and bile acids (BAs).
- Random Forest (RF) Model: A multivariable RF model was developed to evaluate the predictive potential of gut microbiota-derived metabolites for POAF events in both a discovery cohort and a larger validation cohort.
Key Findings
Gut Microbial Alterations:
- Increased Abundance of Actinobacteria and Firmicutes: POAF patients showed significant increases in these phyla compared to non-POAF patients. These bacteria are involved in the metabolism of secondary BAs.
- Microbiota Diversity and Structure: Significant differences in gut microbiota diversity and structure were observed between POAF and non-POAF patients.
Bile Acids (BAs) Metabolism:
- Primary and Secondary BAs: Plasma concentrations of both primary (e.g., cholic acid) and secondary BAs (e.g., deoxycholic acid) were significantly higher in POAF patients.
- Correlation with Gut Microbiota: These elevated BAs were positively correlated with the abundance of Actinobacteria and Firmicutes, indicating a disrupted gut microbiota-bile acid axis in POAF.
Short-Chain Fatty Acids (SCFAs):
- Higher Levels in POAF Patients: Plasma levels of SCFAs such as acetic acid, propionic acid, and isobutyric acid were significantly elevated in POAF patients. These SCFAs have various effects on cardiac function and inflammation.
Inflammation and Oxidative Stress:
- Increased TMAO: Trimethylamine N-oxide (TMAO), a harmful microbial metabolite, was significantly higher in POAF patients and is known to contribute to cardiovascular disease through inflammation and oxidative stress.
- Decreased Antioxidant Metabolites: Levels of arginine and methionine sulfoxide, both antioxidant and anti-inflammatory metabolites, were significantly lower in POAF patients.
Predictive Model for POAF:
- Combination of Secondary BAs: The RF model using five secondary BAs showed high accuracy in predicting POAF, with an area under the curve (AUC) of 0.954 and a correct classification rate (CCR) of 93.3% in the discovery cohort.
- Validation in Larger Cohort: The model also performed well in an independent validation cohort, with an AUC of 0.872 and a CCR of 90.4%, indicating the robustness of these microbial markers as predictive tools for POAF.
The study demonstrated significant alterations in the gut microbiota and its metabolites associated with POAF, particularly focusing on the role of secondary BAs. These findings suggest the gut-heart axis plays a crucial role in the development of POAF and highlight the potential of gut microbiota-derived metabolites as diagnostic biomarkers and therapeutic targets. Further research, including clinical intervention studies, is necessary to validate these findings and explore the underlying mechanisms in greater detail.
Link to the study : https://tinyurl.com/52c84n8d