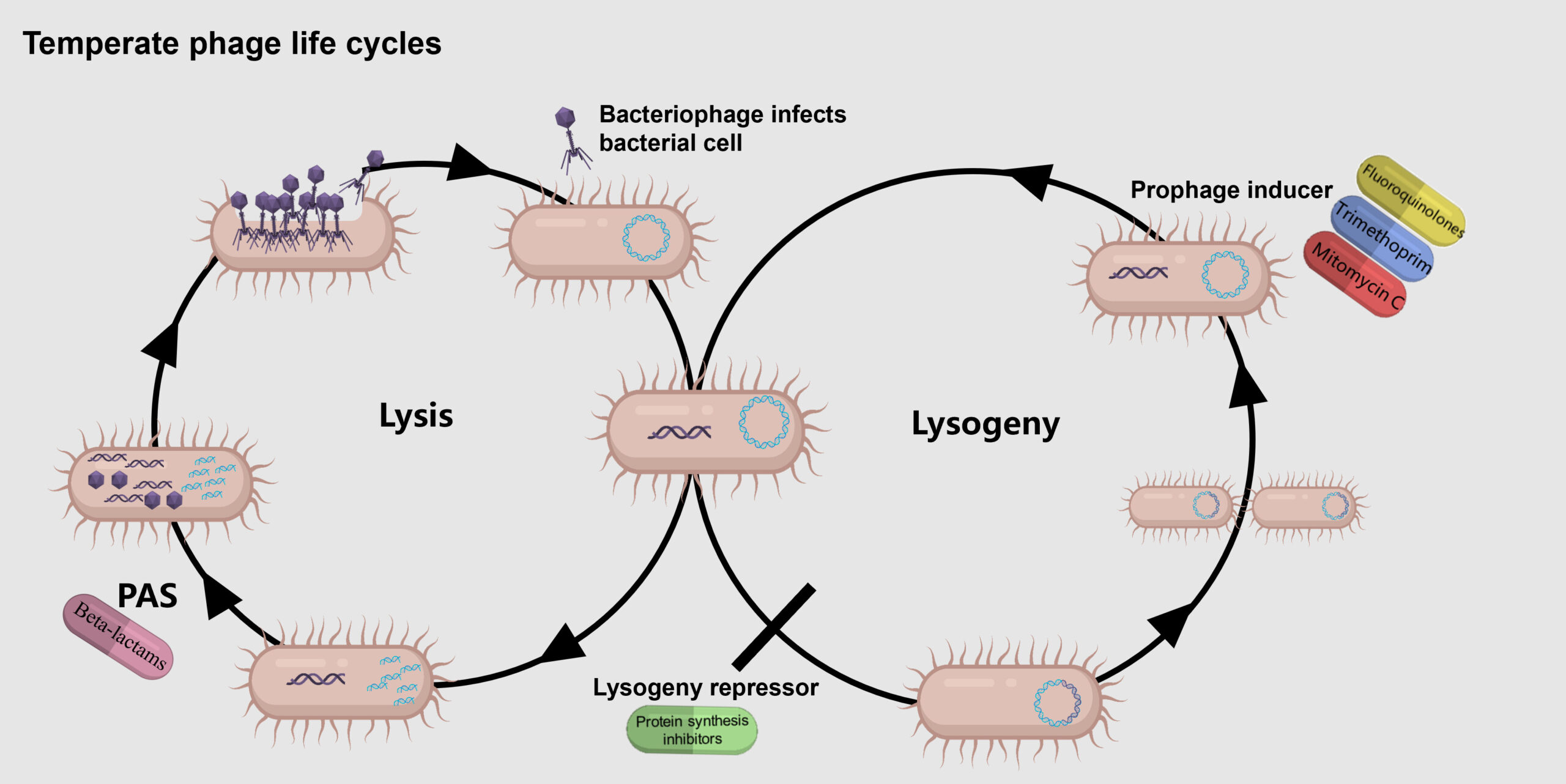
- Temperate Phage-Antibiotic Synergy (tPAS): Recent studies have shown that certain antibiotics can work synergistically with temperate phages to enhance bacterial killing. This effect, known as temperate phage-antibiotic synergy (tPAS), involves interactions with the phage’s decision to lyse the host or integrate into the host genome (lysogeny).
- Objective: The primary aim is to determine if tPAS is generalizable across various antibiotics and to explore the underlying mechanisms, particularly focusing on how different antibiotics influence the lysis-lysogeny decision in phages.
Methodology:
- Phage and Antibiotic Combinations: The E. coli K-12 strain infected with temperate phage HK97 was challenged with 13 different antibiotics from seven classes.
- Checkerboard Assays: This technique was employed to measure the minimum inhibitory concentration (MIC) and assess the synergy between phages and antibiotics.
Key Findings:
Synergy with SOS-Inducing Antibiotics:
- Quinolones: Synergy was observed with nalidixic acid (16-fold reduction in MIC) and ciprofloxacin (8-fold reduction), but weaker with oxolinic acid and levofloxacin. This indicates a variation in the strength of synergy within the quinolone class.
- Mitomycin C and Trimethoprim: These DNA-damaging agents showed significant synergy, leading to drastic reductions in MIC. The effect was reduced in the absence of RecA, a key protein in the SOS response pathway.
- β-Lactams: Inconsistent results were noted, with strong synergy seen with ceftazidime (16-fold reduction) but weak or no synergy with other β-lactams like ampicillin and cefotaxime. This suggests that not all β-lactams induce the SOS response to the same extent.
Synergy with Non-SOS-Inducing Antibiotics:
- Protein Synthesis Inhibitors: Surprisingly, strong synergy was observed with gentamicin, kanamycin, tetracycline, and azithromycin, none of which are known to induce the SOS response. For example, gentamicin reduced the MIC by eightfold.
- Mechanism Analysis: Unlike SOS-inducing antibiotics, the synergy with protein synthesis inhibitors did not depend on RecA and did not result in increased sensitivity of lysogens to antibiotics. Instead, these antibiotics appeared to reduce the frequency of lysogeny and favor lysis.
Mechanistic Insights:
- Lysis-Lysogeny Decision: For gentamicin, the reduction in lysogeny frequency was evident early and persisted, indicating that the antibiotic biases the initial decision towards lysis rather than affecting already formed lysogens.
- Absence of Traditional Induction: The synergy observed did not correlate with traditional phage induction or changes in burst size, suggesting a novel mechanism.
Conclusion:
- Generalizability of tPAS: The study confirms that SOS-inducing antibiotics broadly result in temperate-phage-specific synergy. However, synergy with non-SOS-inducing antibiotics like protein synthesis inhibitors indicates the presence of additional, novel mechanisms influencing the lysis-lysogeny decision.
- Implications for Therapy: Understanding and manipulating the lysis-lysogeny decision with different classes of antibiotics offers new avenues for therapeutic applications, potentially enabling the use of temperate phages in clinical settings.
Importance:
- Lysis-Lysogeny Decision in Biology: This decision is critical as it determines whether phages will destroy their host or integrate and remain dormant, influencing bacterial survival and behavior.
- Phage Therapy: By biasing phages towards lysis, it might be possible to develop new treatments that leverage phages to combat antibiotic-resistant bacteria.
Link to the Study : https://tinyurl.com/4be436pt