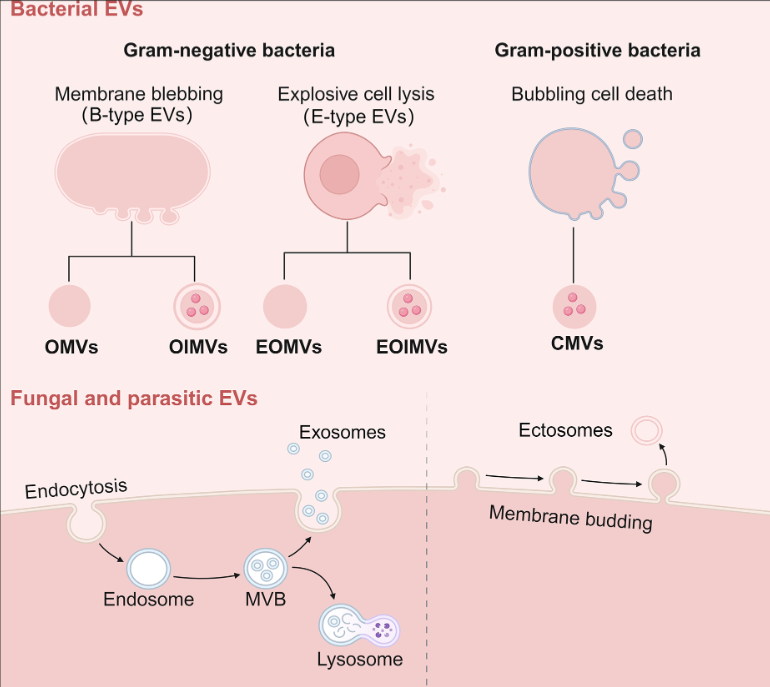
This study delves into the fascinating world of microbial extracellular vesicles (EVs) and their role in antimicrobial resistance. Essentially, EVs are like tiny communication packages that bacteria, fungi, and parasites use to interact with their environment, including other microbes and even antibiotics.
One major finding is that bacterial EVs can act as clever decoys against antibiotics. Imagine EVs as little bodyguards that shield bacteria by either binding to antibiotics directly or encapsulating them inside their membranes. This makes it harder for antibiotics to reach and kill the bacteria.
Key Findings:
- Role of Microbial Extracellular Vesicles (EVs) in Antimicrobial Resistance (AMR): The study explores how EVs released by bacteria, fungi, and parasites contribute to antimicrobial resistance. These EVs can directly or indirectly enhance resistance to antibiotics, making it harder to treat infections.
- Mechanisms of EV-Mediated Resistance: EVs from bacteria can act as decoys to bind or encapsulate antibiotics, degrade antibiotics using enzymes, and transfer resistance genes to other cells. Fungal EVs primarily contribute to resistance by aiding in biofilm formation or cell wall repair.
- Application of Bacterial EVs in Antimicrobial Therapy: Bacterial EVs show promise in antimicrobial therapy. They possess natural antibacterial activity, can be loaded with antibiotics for targeted delivery, and mimic bacterial membranes for assessing antibiotic permeability.
- Decoy Action of EVs: EVs from bacteria can “trick” antibiotics by binding to them or encapsulating them, reducing their effectiveness against the bacteria.
- Enzymatic Degradation: Bacterial EVs can carry enzymes like β-lactamases, which break down antibiotics, providing protection to the bacteria.
- Gene Transfer: EVs can transfer resistance genes between bacteria, spreading resistance to different species and strains.
- Application in Therapy: Bacterial EVs offer potential in antimicrobial therapy due to their natural antibacterial activity and ability to deliver antibiotics effectively.
There are still some technical hurdles to overcome, like how to produce EVs on a large scale and how to tweak their surfaces for specific tasks. But the potential benefits are clear: a deeper understanding of EVs could lead to groundbreaking advancements in antimicrobial therapy.
Link to the article : https://tinyurl.com/ywre2uuc