💡 The study investigates the intricate communication system known as the microbiome-gut-brain axis, exploring its potential influence on brain health and related diseases. Focusing on the bidirectional flow of metabolites and extracellular vesicles (EVs) from gut microbes to the central nervous system (CNS), the research aims to understand the underlying mechanisms.
A gut-brain axis chip was developed, featuring microfluidic-based models for the gut and brain, utilizing human induced pluripotent stem cells (iPSCs)-derived neurons. The report provides novel insights into the impact of microbial-derived substances on neurodevelopment and neurodegenerative disorders.
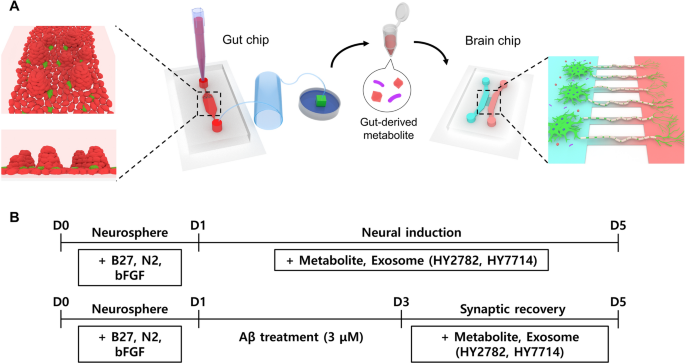
📍 Microfluidic Gut-Brain Axis Chip: The experimental setup includes a gut chip with a large channel for Caco-2 cell culture and a brain chip comprising channels for human induced neural stem cells (iNSCs) and neural stem cell axons. Microfluidic conditions in the gut chip resulted in robust differentiation and polarization of Caco-2 cells, closely mimicking in vivo intestinal structure. The introduction of microbiota demonstrated stable colonization without compromising epithelial integrity, affirming the effectiveness of continuous dynamic culture conditions.
📍 Microbe-Derived Metabolites Impact on Neural Differentiation: Microbe-derived metabolites were observed to significantly enhance neurite length, maturation, and synaptic plasticity in iNSCs-derived neurons within the gut-brain axis chip.
Metabolites derived from specific bacterial strains, such as Hy2782 and Hy7714, exhibited varying but promising effects on neurogenesis.
The expression of Tuj1, a neural marker, increased significantly, suggesting the crucial role of microbial metabolites in promoting early neural development.
📍 Microbe-Derived Metabolites and Synaptic Protein Expression: The study delves into the expression of synapse-related proteins (PSD95 and GAP43) under the influence of microbial metabolites.
Observations revealed a marked increase in the expression of these proteins, indicating the potential of metabolites to influence synaptic plasticity and contribute to the maturation of neural structures.
The findings suggest a pivotal role for microbial-derived metabolites in fostering the differentiation of neural progenitor cells into mature and functional neurons.
📍 Microbe-Derived Exosomes Influence Neural Differentiation and Synaptic Plasticity: Examining the impact of microbe-derived exosomes on neural differentiation, the study found a significant increase in neurite length and the expression of neural markers.
Furthermore, synaptic plasticity-related proteins (GAP43 and PSD95) exhibited elevated expression, emphasizing the role of exosomes in promoting synaptic maturation during early neurogenesis.
📍 Microbial Impact on Alzheimer’s Disease Model:
In the context of an Alzheimer’s disease model, the study explored the therapeutic potential of microbe-derived metabolites and exosomes. Despite amyloid-beta treatment inducing Alzheimer’s-like conditions, the metabolite- and exosome-treated groups displayed enhanced axonal growth, synaptic activity, and protein expression associated with synaptic plasticity. This suggests a potential protective effect against neurodegenerative diseases, particularly Alzheimer’s.
⭕ The research underscores the profound impact of microbial-derived metabolites and exosomes on neural development and neurodegenerative diseases within the context of the gut-brain axis chip. These findings open avenues for future investigations into the therapeutic potential of microbe-derived substances in addressing neurodevelopmental and neurodegenerative disorders.
The gut-brain axis chip emerges as a valuable tool for studying the dynamic interplay between gut microbiota and neural cells, offering insights that could revolutionize our understanding of neurological health and disease.
Link to the article : http://tinyurl.com/yc2swdzp