💡 Prenatal exposure to intrauterine inflammation (IUI) is a critical event in the pathophysiology of preterm birth (PTB), contributing to increased rates of neurodevelopmental disorders. This study explores the intricate relationship between gut microbiota, metabolite profiles, and LPS-induced IUI in a mouse model, shedding light on potential mechanisms underlying PTB.
📍 Methodology:
📌 Mouse Model and Verification: Established IUI-exposed PTB mouse models were verified based on PTB rates and perinatal adverse reactions. LPS-induced IUI significantly increased PTB rates, apoptosis, and inflammation in placental tissue samples.
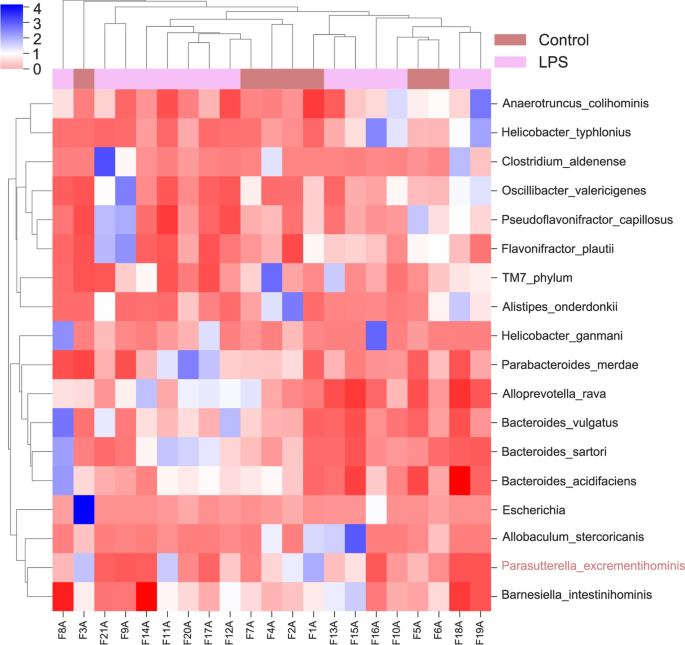
📌 Microbiota Analysis: LPS-induced IUI altered species abundance distribution without significant changes in richness and evenness. Dysbiosis of gut microorganisms, particularly reduced abundance of 𝘗𝘢𝘳𝘢𝘴𝘶𝘵𝘵𝘦𝘳𝘦𝘭𝘭𝘢 𝘦𝘹𝘤𝘳𝘦𝘮𝘦𝘯𝘵𝘪𝘩𝘰𝘮𝘪𝘯𝘪𝘴, was associated with inflammatory and metabolic abnormalities.
📌 Metabolomics Analysis: Non-targeted metabolomics analysis revealed altered metabolite profiles in preterm mice. Differential metabolites were associated with signaling pathways, including pyruvate metabolism.
📌 Correlation Studies: Identified a significant positive correlation between 𝘗𝘢𝘳𝘢𝘴𝘶𝘵𝘵𝘦𝘳𝘦𝘭𝘭𝘢 𝘦𝘹𝘤𝘳𝘦𝘮𝘦𝘯𝘵𝘪𝘩𝘰𝘮𝘪𝘯𝘪𝘴 and specific metabolites (e.g., pyruvic acid, Nb-p-coumaroyltryptamine).
📌 Intervention and Treatment: Pyruvic acid treatment showed significant improvement in LPS-induced IUI phenotypes. Pyruvic acid intervention correlated with decreased PTB rates, apoptosis, and inflammation in placenta tissue samples.
📍 Key Findings:
📌 Association with PTB: LPS-induced IUI was a crucial pre-event of PTB, associated with increased PTB rates, apoptosis, and inflammation in placental tissues. Elevated TNF-α and IL-6 levels indicated inflammatory responses linked to premature labor.
📌 Microbiota Dysbiosis: Dysbiosis in gut microbiota, particularly reduced 𝘗𝘢𝘳𝘢𝘴𝘶𝘵𝘵𝘦𝘳𝘦𝘭𝘭𝘢 𝘦𝘹𝘤𝘳𝘦𝘮𝘦𝘯𝘵𝘪𝘩𝘰𝘮𝘪𝘯𝘪𝘴 abundance, was observed in LPS-induced IUI models. Dysbiosis was suggested as a potential risk factor for inflammatory and metabolic abnormalities.
📌 Oxidative Stress and PTB: Increased levels of oxidative stress markers in placentas of model mice indicated a link between oxidative stress and PTB.
Previous research suggested that antioxidants might have protective effects on PTB and unfavorable birth outcomes.
📌 Metabolite Alterations: Altered metabolite profiles, especially associated with pyruvate metabolism, highlighted the role of metabolites in the pathophysiology of PTB. Specific metabolites like pyruvic acid were correlated with gut microbiota changes and showed potential in mitigating adverse effects.
📌 Pyruvic Acid Intervention: Pyruvic acid treatment demonstrated a positive impact on LPS-induced IUI phenotypes, including decreased PTB rates and inflammation. Correlation studies suggested a potential mediating role of pyruvic acid in the interaction between gut microbiota and LPS-induced IUI.
📍 This study unveils a complex interplay among gut microbiota dysbiosis, altered metabolite profiles, and LPS-induced IUI in the pathophysiology of PTB. The findings underscore the potential role of gut microbiota and metabolites, such as pyruvic acid, in influencing adverse pregnancy outcomes.
Link to the article : http://tinyurl.com/475saf2h