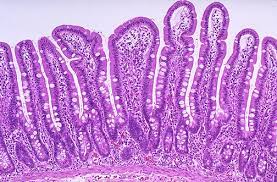
In the intricate landscape of the gastrointestinal tract, the colon takes the spotlight as an active center of microbial activity, where the dynamic interplay between the mucosal layers and the resident microbiota unfolds. At the heart of this symbiotic relationship lies the colon mucus layer, a complex structure finely tuned to facilitate the coexistence of the host and its microbial inhabitants.
Picture the colon mucus layer as a guardian with two distinct realms—a formidable inner layer, impervious to bacterial intrusion, and an expansive outer layer, welcoming the colonization of commensal microbes. MUC2, a glycoprotein with an abundant O-glycan tapestry, is at the molecular forefront of this defense. The designers of this glycosylated barrier, the goblet cells, are skilled in the gut, producing a haven for the commensal non-pathogenic microbiota within the outer mucus layer.
Commensal bacteria, aptly described as “O-glycan degraders” or “mucin utilizers,” possess a repertoire of glycan-degrading enzymes. They cleave and utilize mucin O-glycans as an energy source, establishing a mutually beneficial relationship without disturbing the integrity of the inner mucus layer. In contrast, pathogens possess proteases capable of dissolving the protective inner mucus layer, paving the way for invasive bacterial tissue invasion.
Within the mucus milieu, highly glycosylated mucins present multiple O-glycan epitopes, acting as attachment points for commensal bacteria. Certain species of the microbiota produce proteins that bind to these O-glycans and facilitate interactions that are essential for colonization. Notably, changes in the composition of the microbiota have been associated with modifications in O-glycosylation, highlighting the complex interplay between the microbe and the host.
The genetic coordination of this harmonious connection is encapsulated in polysaccharide utilization loci (PULs), specific genomic regions encoding genes for recognizing, degrading, taking up, and sensing glycans. For example, B. theta has dedicated 18% of its genome to 88 PULs, of which at least 16 PULs are targeting the utilization of mucin O-glycans. Complex glycans are recognized and broken down by a variety of enzymes, including carbohydrate-binding modules, sulfatases, glycoside hydrolases, and proteases.
The sugar code embedded within mucins emerges as a critical determinant in the dynamic interplay between host and microbe. The mucus layer acts as a protector in both health and illness, affecting intestinal barrier function and preserving stability. Deprivation of dietary polysaccharides creates vulnerabilities that cause the intestinal wall to erode the colonic mucous layer and increased susceptibility to enteric pathogens.
📍 Delving into specific health contexts, the symbiotic relationship between mucins and microbes unveils its relevance in various diseases:
📌 Necrotising Enterocolitis (NEC): Affecting premature infants, NEC involves compromised mucosa, leading to bacterial binding and mucosal infection. Glycan-based prebiotics show promise in influencing the microbiota and fortifying the mucosal defensive barrier.
📌 Inflammatory Bowel Disease (IBD): Embracing conditions like ulcerative colitis and Crohn’s Disease, IBD manifests as mucosal damage. Alterations in mucin glycosylation, including changes in MUC gene expression, offer insights into the intricate connection between glycobiology and disease pathology.
📌 Colorectal Cancer: The journey from adenomas to carcinomas in colorectal cancer maintains a link to mucin-based events. Changes in MUC2 and glycosylation patterns provide a molecular landscape of colorectal cancer, hinting at its association with mucin alterations.
📍 Several mechanisms contribute to immune homeostasis at the mucosal interface:
📌 Tolerance to Commensal Bacteria: The mucus barrier establishes and maintains tolerance to the vast community of commensal bacteria, acting as a physical shield and containing factors like secretory IgA that contribute to immune tolerance.
📌 Antimicrobial Defense: Antimicrobial components within the mucus layer, including peptides and immunoglobulins, defend against potential pathogens, controlling the composition of the gut microbiota.
📌 Regulation of Immune Responses: The gut microbiota, shaped by the mucus barrier, influences the development and function of the immune system, orchestrating a balanced immune reaction.
📌 Induction of Immune Tolerance: Proximity to the mucus layer facilitates the gut microbiota’s role in inducing immune tolerance, contributing to the development of regulatory T cells (Tregs) and other immune regulatory mechanisms.
📌 Maintenance of Gut Homeostasis: The mucus barrier and gut microbiota collectively contribute to the overall homeostasis of the gut environment. They uphold the integrity of the epithelial barrier, regulate inflammatory responses, and support the general health of the gastrointestinal tract.
As we unravel the secrets encrypted within the mucins, a deeper understanding of gastrointestinal health emerges. In addition to providing insights into the delicate balance of this symbiotic connection, the complex interaction between host and microbiota, guided by the language of glycans, may also open up new possibilities for targeted therapeutic interventions and individualized approaches to gastrointestinal health.
Further reading :
Intestinal mucus and their glycans: A habitat for thriving microbiota : http://tinyurl.com/5xusy3ea
Mucin glycan foraging in the human gut microbiome : http://tinyurl.com/43zpwuch
Glycan processing in gut microbiomes : http://tinyurl.com/yc4datt7
Mucosal glycan degradation of the host by the gut microbiota : http://tinyurl.com/2wtyhf72
Slimy partners: the mucus barrier and gut microbiome in ulcerative colitis : http://tinyurl.com/2fvbv4yf