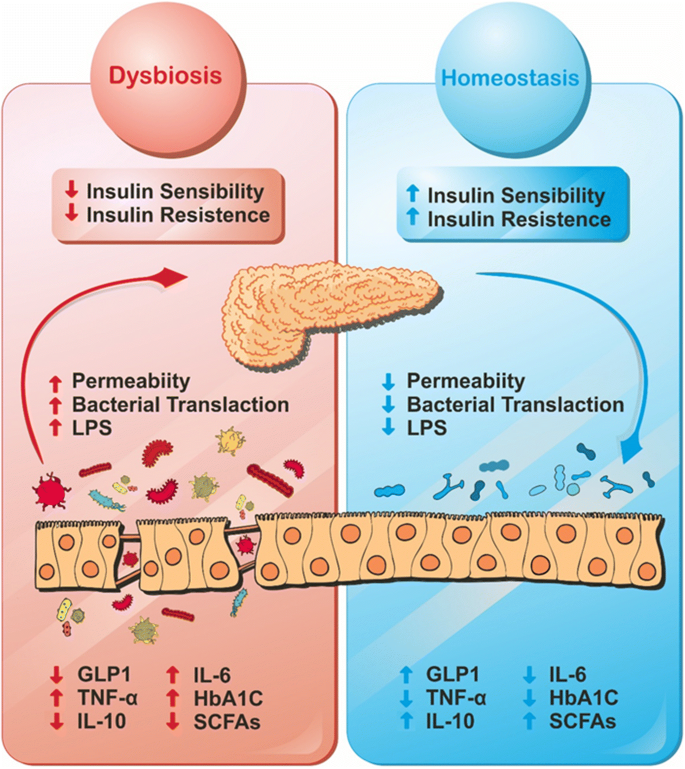
In the intricate landscape of metabolic diseases, the gut microbiota emerges as a key player, managing a harmony of dysbiotic alterations that fuel chronic inflammation in metabolic tissues. A complicated interaction between immunological reactions, nutritional factors, and molecular signaling reveals the relationship between diabetes and the gut microbiota.
Dysbiosis-related metabolic illnesses have a dysregulated immunological ensemble due to commensal bacteria and bacterial fragments that infiltrate into metabolic tissues and cause a series of pro-inflammatory reactions. By undermining the integrity of the intestinal barrier, the high-fat diet (HFD), a major player in metabolic endotoxemia, intensifies the events. This food allergen increases levels of lipopolysaccharide (LPS), a powerful inflammatory mediator. In the context of inflammation, toll-like receptors (TLRs), particularly the sentinel TLR-4, play crucial roles and open the door for the rise of insulin resistance.
The gut’s defense, represented by the mucus layer, undergoes extreme thinning under the influence of dysbiotic conditions induced by the HFD. This compromise becomes an arena for conflict where immune members like T helper 17 cells and interleukin-22, crucial for mucosal barrier integrity, find themselves outnumbered and overpowered.
In the therapeutic space, probiotics emerge as protectors, with Bifidobacteria and Akkermansia leading the charge for treatment. By lowering bacterial adhesion and translocation, these microbial allies suppress tissue inflammation and insulin resistance, demonstrating their effectiveness. Their noble purpose also includes the manipulation of gut microbial structure, which is a calculated move towards achieving metabolic homeostasis.
Prebiotics are armed with polyphenolic shields. Similar to guardians of dietary virtue, these substances provide defense against the attack of diet-induced metabolic syndrome. The key to their success is encouraging the growth of advantageous bacteria, such as the resilient Akkermansia.
MAMPs, or molecular patterns linked with bacteria, are responsible for the concealed movement of pathogens known as bacterial translocation. In this instance of microbial espionage, NOD1 and NOD2 function as peptidoglycan sensors, whereas receptors such as CD14 bind bacterial LPS. Enter the polyphenols and bacterial muramyl dipeptide, strategic tools that target these receptors, unleashing anti-inflammatory effects and restoring the harmony of insulin signaling.
Moving into the domain of nutrient-sensing receptors, the G-protein coupled receptors (GPCRs), the metabolites produced from dietary polysaccharides, short-chain fatty acids, activate GPR41/FFAR3 and GPR43/FFAR2, generating effects on intestinal function, energy homeostasis, and metabolic regulation. Dietary messengers that are accessible to the microbiota may be used to influence these receptors, providing an effective way of treating metabolic disorders.
GPR119, which is capable of identifying long-chain fatty acids, appears to be a validated therapeutic target in Type 2 Diabetes. This receptor takes on the role of mediator and interpreter of the release of insulin that is triggered by glucose. The revelation of structural and functional connections between human ligands for GPR119 and their microbiota-encoded counterparts opens a novel perspective on antidiabetic agents, a potential revolution in the pharmacopeia of diabetes.