Your gut might hold secrets to Alzheimer’s disease (AD), and neuroinflammation seems to be the chief suspect!
When infections come into play, a plethora of cell signaling pathways get activated, leading to inflammation. If infectious microorganisms break through the blood-brain barrier, it can result in neuronal death and share eerily similar hallmarks with AD. Inflammation is a necessary response for protection, tissue repair, and getting rid of waste. In the AD playbook, neuroinflammation is a significant player, with activated microglia and astrocytes taking center stage. Microglia, brain’s immune warriors, are vital in the neuroinflammation saga. They can morph into different phenotypes depending on the brain’s signals and express innate immune receptors. When these receptors activate, the microglia go into action, producing inflammatory mediators, including reactive oxygen species, nitric oxide, and cytokines. These activated microglia, in the presence of Aβ, migrate to plaques, gobbling up Aβ and triggering an inflammatory response. It’s like a cellular warzone, with pattern recognition receptors as the generals.
But don’t forget about astrocytes, the brain’s unsung heroes, five times more abundant than neurons. These cells help maintain the CNS’s integrity, but when Aβ accumulates, they transform into activated astrocytes. These “super” astrocytes overproduce cytokines and generate oxidative stress, adding more fuel to the fire.
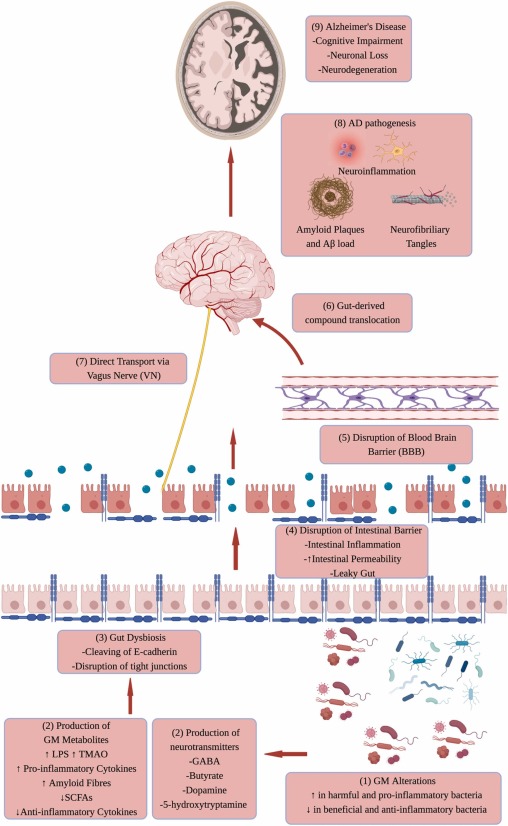
A growing body of evidence suggests that the gut microbiota, the community of microorganisms residing in our digestive system, plays a significant role in the development of the brain. This relationship is often referred to as the “brain-gut-microbiome axis.” It’s a two-way street, where the brain, gut, and gut bacteria influence each other. Let’s dive into the details of this fascinating interplay.
The gut microbiota has the power to influence the brain-gut connection through various mechanisms, including immunological, neuroendocrine, and direct neural pathways. These microorganisms can promote local and systemic inflammation through the release of compounds like lipopolysaccharides (LPS) from pathogenic bacteria and the production of pro-inflammatory cytokines. Moreover, they are capable of generating neurotransmitters and neuromodulators, such as short-chain fatty acids (SCFAs), histamine, serotonin, and gamma-aminobutyric acid (GABA). On the flip side, they can also create neurotoxic metabolites like d-lactic acid and ammonia. These signaling molecules travel through the circulatory system and lymphatic system to reach the central nervous system (CNS), affecting behavior, brain plasticity, and cognitive function. This underscores the gut microbiota’s pivotal role in CNS development, function, and the pathophysiology of chronic brain diseases.
A recent study shed light on how molecules secreted by the gut microbiota contribute to brain inflammation. In the CNS, microglia and astrocytes, two types of immune cells, communicate using molecules like vascular endothelial growth factor B (VEGF-B) and transforming growth factor alpha (TGF-α) to regulate neuroinflammation. This communication is facilitated by a ligand-activated transcription factor called aryl hydrocarbon receptor (AHR), traditionally associated with processing environmental toxins. Interestingly, the deletion of AHR in microglia increased experimental autoimmune encephalitis (EAE) severity, an inflammatory disease in the CNS. AHR’s activation in microglia seems to inhibit CNS inflammation. The study also revealed AHR’s direct involvement in the expression of TGF-α and VEGF-B, which, in turn, affect the inflammatory responses of astrocytes. While TGF-α reduces inflammation in astrocytes, VEGF-B does the opposite. This intricate interplay showcases the profound effects of the gut microbiota on brain inflammation.
Moreover, research demonstrated that supplementing mice with specific tryptophan metabolites and bacterial enzymes reduced CNS inflammation. These molecules can also potentially disrupt communication between specific neural structures of the brain, like dopaminergic neurons.
The connection between the gut microbiota and Alzheimer’s disease (AD) was exemplified in a study using transgenic mouse models. Shifting the gut microbiota diversity of APP transgenic mice led to changes in cerebral amyloid-beta (Aβ) pathology. When germ-free APP transgenic mice were colonized with gut microbiota from conventionally raised APP transgenic mice, cerebral Aβ pathology increased. These findings emphasize the significant role of the gut microbiota in AD pathology.
Intriguingly, many bacteria can synthesize and release neurotransmitters, neuromodulators, and neuropeptides. This hints at the gut microbiota’s involvement in the development of AD pathology. Additionally, chronic inflammation in the gut may disrupt the blood-brain barrier, leading to increased permeability, the generation of pro-inflammatory cytokines, and elevated levels of LPS. In fact, AD patients often exhibit higher LPS levels in their blood. Peripheral inflammation, such as colitis, can exacerbate neuroinflammation and neurodegeneration.
The presence of bacterial amyloid, a component of the gut microbiota, in the CNS contributes to Aβ accumulation, which is closely linked to AD. This suggests that modulating the gut microbiota could be a potential strategy to prevent or reduce the risk of AD.
It’s clear that the gut microbiota influences neuroinflammation, which, in turn, has significant implications for the development of neurodegenerative disorders like AD. The delicate balance in gut microbial diversity can lead to detrimental effects on the brain. Changes in gut composition may help delay or prevent the progression of these disorders. Promising research points to probiotics and specific diets as potential interventions. Understanding these molecular pathways will pave the way for better treatments for neurodegenerative diseases.
In conclusion, the intricate relationship between the gut microbiota and the brain offers a deeper understanding of the complex processes at play in neuroinflammation and neurodegeneration, particularly in the context of Alzheimer’s disease. These findings hold great promise for future therapeutic interventions and shed light on the critical role of gut health in brain function and disease.
Refrences :
Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease : https://tinyurl.com/5n6h2jfv
Targeting gut microbiota to alleviate neuroinflammation in Alzheimer’s disease : https://tinyurl.com/2bjr8ndv
The Microbiota–Gut–Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? : https://tinyurl.com/44hvtj3a