💡 This comprehensive review explores the intricate interplay between the gut microbiome and the central nervous system (CNS), with a specific focus on microglial cells.
Notably, prenatal maternal microbiome and diet are identified as early influencers of microglial activity, which has profound implications for long-term neuroarchitecture and the risk of neurodevelopmental disorders.
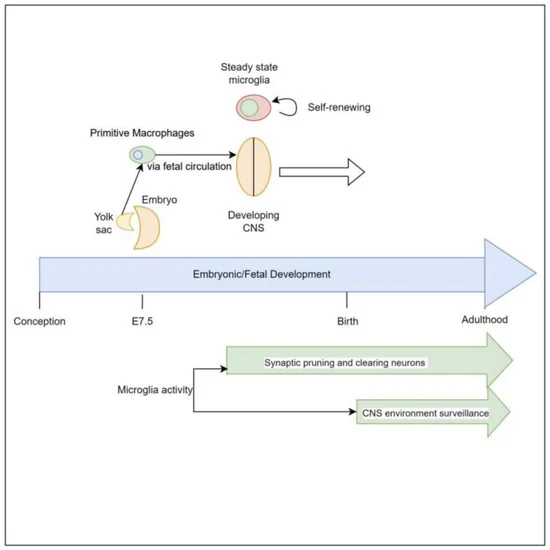
Microglia as Central Figures:
Microglia, as crucial components of the CNS, serve various functions in neurodevelopment and neuroinflammation. They exhibit diverse responses, including activation, deactivation, proliferation, and senescence, depending on the specific brain regions and conditions they encounter. The CNS functions are influenced by signals originating not only from within the CNS but also from external factors. The gut microbiome, a complex ecosystem of trillions of microorganisms, actively contributes to host health. It aids in maturing the intestinal mucosa, facilitating nutrient absorption, and producing various metabolites with systemic and organ-specific physiological effects.
Gut Microbiome’s Influence on Normal Neurodevelopment:
Studies, including those on germ-free (GF) mice, demonstrate that the absence of normal gut microbiota can lead to behavioral changes and increased motor activity. Restoration of gut microbiota in GF mice restores normal behavior, highlighting the gut microbiome’s role in influencing neurodevelopment. Additionally, metabolites produced by gut microbiota, such as short-chain fatty acids (SCFAs), impact neurodevelopment. Research in mice has shown that SCFA supplementation improves motor function recovery following thrombotic stroke, emphasizing the gut microbiome’s role in post-stroke recovery.
Influence of Microglial Cells on Neuroinflammation:
Microglia have the capacity to modulate neuroinflammation by releasing both pro- and anti-inflammatory chemokines and cytokines. Dysregulation of these cytokine pathways can lead to neuropsychiatric symptoms and altered signaling in the CNS, particularly in mice predisposed to immune dysfunction. Disruption of the blood-brain barrier (BBB) by factors such as inflammatory markers like IL-1, TNF-α, and IL-6 can result in the recruitment of microglia and astrocytes, leading to cytotoxicity.
Interplay Between Gut Microbiome, Microglia, and CNS:
Microbe-associated molecular proteins produced by gut bacteria travel through the intestinal wall and activate macrophages, leading to the release of pro-inflammatory cytokines. These cytokines enter the bloodstream and activate the vagus nerve tract, carrying signals to the CNS. T-cells with the ability to interact with microglia in the brain further influence neuroinflammation. This complex network demonstrates the direct impact of inflammatory markers on neuromodulation.
Prenatal Influences and Maternal Microbiome:
Maternal diet and microbiome play a crucial role in influencing offspring’s lifelong health outcomes, including immune system activation, neurological development, and metabolism. Research links maternal microbiome to immune system activation in offspring and allergy development. Exposure to environmental antigens and maternal microbiome diversity are necessary for a healthy immune system. The maternal microbiome and breast milk microbiota affect fetal immune system and allergy development. Various factors such as the “farm effect,” antibiotic use, dietary fiber, and psychological stress can alter maternal and fetal microbiomes and influence inflammatory responses in infants.
Impact on Neurodevelopmental Disorders:
Numerous studies have linked gut dysbiosis to neurological disorders, including autism spectrum disorder (ASD), schizophrenia, attention deficit hyperactivity disorder (ADHD), anxiety, depression, and epilepsy. Alterations in maternal microbiome and prenatal exposure to inflammatory markers, in conditions such as maternal immune activation, have been associated with neurodevelopmental disorders. Studies demonstrate changes in neuronal gene expression, dendritic spine formation, and dopaminergic system defects in offspring due to maternal immune activation, potentially leading to ASD and other neuropsychiatric conditions.
🔴 The Gut-Brain axis, mediated by microglial cells, shapes normal neurodevelopment and neuroinflammation, with implications for both prenatal and postnatal life.
Link to the article: https://tinyurl.com/2p89598w