💡 This study analyzed the impact of the FilmArray Blood Culture Identification Panel – an amplification-based diagnostic test – on the treatment and clinical outcomes of bloodstream infections, especially those linked to multidrug-resistant microorganisms.
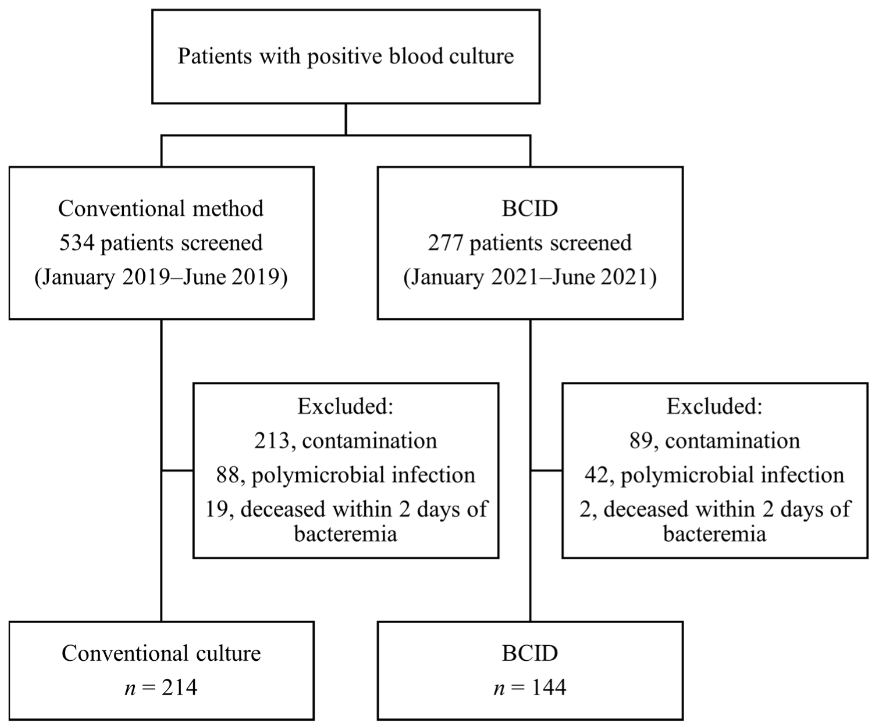
📌 This retrospective study compared patients who underwent BCID testing with a historical control group that used conventional culture testing methods. Patients with bacteremia caused by carbapenem-resistant Enterobacterales and vancomycin-resistant enterococci were specifically analyzed.
📌 A total of 144 patients who underwent BCID testing and 214 patients who underwent conventional cultures were included. The 30-day mortality rate, time to effective antibiotic administration, and time to appropriate antibiotic administration did not differ significantly between the groups.
1. The BCID panel did not significantly change 30-day mortality rates or time to antibiotic administration.
2. BCID shortened the time to effective treatment for specific drug-resistant infections.
3. There was no observed change in 30-day mortality after adjusting for relevant variables.
4. Clinical outcomes didn’t improve significantly across patient subgroups with BCID testing.
5. BCID’s clinical impact was limited due to partial antimicrobial susceptibility information and lack of real-time management.
📌 BCID reduced the time to administration of effective antibiotics in cases of CRE (39 h vs. 93 h) and VRE (50 h vs. 92 h) bacteremia. However, BCID was not significantly associated with 30-day mortality after adjusting for the Pitt bacteremia score and the Charlson comorbidity index.
📌 Overall, the use of BCID testing did not affect 30-day mortality or the overall clinical outcomes of bloodstream infections. However, it was beneficial in reducing the time to deliver effective antibiotic treatment for infections caused by certain multidrug-resistant organisms.
Link to the article : https://tinyurl.com/yx35span