💡 This study focuses on understanding resistance to the Bac7 1-22 derivative in multidrug-resistant E. coli isolated from urinary tract infections.
📌 Bac7 1 -22 is a proline-rich antimicrobial peptide having a unique mechanism of action. It inhibits protein synthesis by interacting with the bacterial ribosome, reducing the risk of bacterial resistance development.
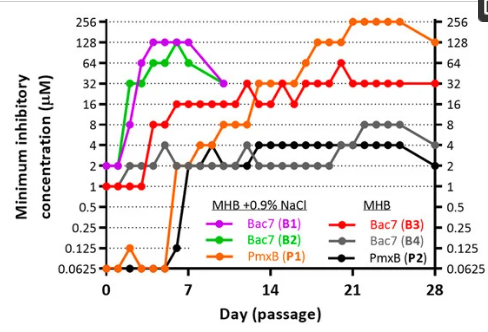
📌 Researchers developed three Bac71-22-resistant strains from a multidrug-resistant E. coli isolate using four weeks of serial passaging, seen by a minimum 16-fold increase in Minimal Inhibitory Concentrations.
📌 They conducted the experiment in varying (both salted and unsalted) media and used different antimicrobial peptides and traditional antibiotics to assess potential cross-resistance.
📌 In a salt-containing medium, bacterial resistance to Bac71-22 was mediated by the inactivation of SbmA transporter. In the absence of salt, the dynamics and main molecular targets under selection pressure were affected. Specifically, a mutation in the WaaP kinase leading to amino acid substitution N159H was observed.
📌 The mutation in WaaP kinase was linked to reduced bacterial susceptibility to both Bac71-22 and polymyxin B. Both sbmA inactivation and waaP mutations result in increased bacterial resistance to Bac7.
📌 Bac7 acts by forming interactions with bacterial ribosomes that significantly reduce bacterial resistance development, but these interactions are affected if the bacteria’s internal structure and membrane composition are altered due to the mentioned mutations.
📌 When conducting antimicrobial screenings with various AMPs and conventional antibiotics, no significant cross-resistance effects were identified in the selected strains.
📍 The study’s insights into bacterial resistance mechanisms may guide antibiotic stewardship strategies by highlighting how optimal use of antibiotics can counter resistances.
It also introduces Proline-rich Antimicrobial Peptides as a potential new class of antibiotics in managing drug-resistant infections.
Link to the article : https://bit.ly/3rWdEuX