Non-Alcoholic Fatty Liver Disease (NAFLD) is a prevalent metabolic disorder characterized by hepatic steatosis, which can progress to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC). Intestinal barrier dysfunction and dysbiosis of gut microbiota have been identified as critical factors in the pathogenesis and progression of NAFLD.
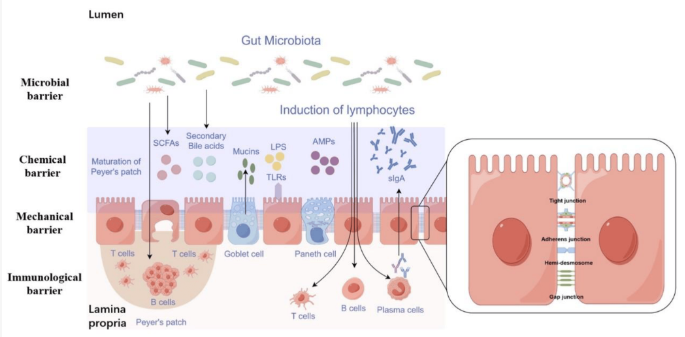
Intestinal Barrier Structure and Function: The intestinal barrier serves as a semi-permeable structure, preventing the entry of harmful substances and microorganisms while facilitating nutrient absorption and immune surveillance. It consists of the gut microbiota, mucus layer, epithelial cells monolayer, and immune cells within the lamina propria.
Mechanisms of Intestinal Barrier Dysfunction in NAFLD:
- Mechanical Barrier: Tight junctions (TJs) and adherens junctions (AJs) maintain intestinal permeability. Alterations in TJ proteins like Claudin, Occludin, and Zonula Occludens-1 (ZO-1) contribute to increased permeability in NAFLD.
- Chemical Barrier: Components like mucus, bile acids, and antimicrobial peptides protect the intestinal lining. Dysregulation of bile acids and antimicrobial peptides can compromise barrier function.
- Immunological Barrier: Gut-associated lymphatic tissue (GALT) and secretory IgA (sIgA) play vital roles in immune defense. Decreased sIgA levels weaken the immune barrier in NAFLD.
- Microbial Barrier: Gut microbiota produce metabolites and enzymes that regulate barrier function. Dysbiosis leads to altered microbial communities, exacerbating intestinal permeability.
Assessment of Intestinal Barrier Dysfunction: Various methods, including exogenous inert probes, biomarkers like zonulin, calprotectin, fatty acid binding proteins (FABPs), citrulline, and trefoil factor 3 (TFF3), and in vitro models such as Caco-2 cell monocultures, co-culture models, and organoids, are employed to assess intestinal barrier integrity in NAFLD.
Key Scientific Findings:
- Increased Intestinal Permeability: Studies have demonstrated elevated intestinal permeability in NAFLD patients, correlated with disease severity. This permeability is associated with disruptions in tight junction proteins like ZO-1 and alterations in microbial communities.
- Biomarkers of Barrier Dysfunction: Biomarkers such as zonulin, calprotectin, FABPs, citrulline, and TFF3 show promise in assessing intestinal barrier dysfunction in NAFLD. Elevated levels of these markers are indicative of increased permeability and disease progression.
- Role of Gut Microbiota: Dysbiosis in gut microbiota composition, characterized by increased Proteobacteria and decreased beneficial bacteria, contributes to intestinal barrier dysfunction in NAFLD. Microbial metabolites and enzymes further exacerbate barrier disruption.
- In vitro Models for Research: Caco-2 cell monocultures, co-culture models, and organoids provide valuable tools for studying intestinal barrier function in NAFLD. These models offer insights into cellular mechanisms and allow for drug screening and disease modeling.
Intestinal barrier dysfunction and dysbiosis of gut microbiota play pivotal roles in the pathogenesis and progression of NAFLD. Understanding the mechanisms underlying barrier dysfunction and employing reliable assessment methods are crucial for developing targeted therapeutic interventions to mitigate NAFLD progression and its associated complications.
Link to the study : https://tinyurl.com/mrj2ppxp