We acknowledge the description of Cefepime-Enmetazobactam which is expected to be brought to the market by Advanz Pharma in Europe.
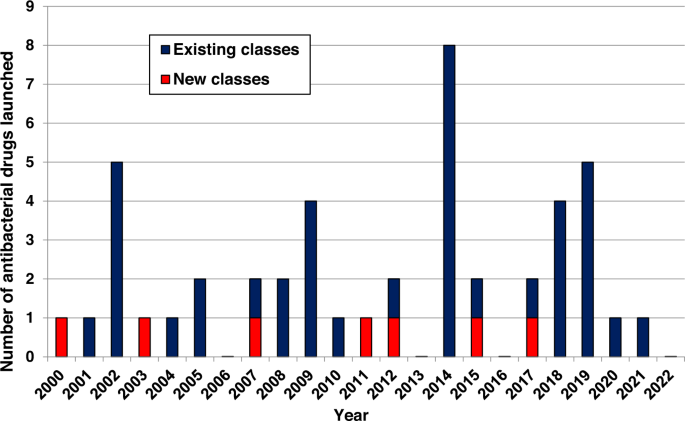
💡 The authors monitored antibacterial drug development since 2011. They systematically analysed the existing and upcoming funding, policy, and legislative initiatives designed to revive antibacterial R&D. Further, they reviewed patents from 2010-2021 that focus on compounds with activity against multi-drug resistant Gram-ve bacteria, antibacterial combinations, discovery strategies, and cataloged the small molecule antibacterial drugs launched since January 2012.
📌 The shape of the antibacterial pipeline has changed since the first analysis in 2011. At the front-end of the pipeline, there are now more than double the number of phase-| candidates (26) compared to 11 in 2015.
Small molecule non-traditional antibacterial candidates are in active development: fluorothyazinone, dovramilast, BVL-GSK098, GSK3882347 and ALS4. Due to these increasing number of compounds with novel pharmacophores and targets in the pipeline, it is more likely that novel antibacterial drug classes will enter the clinic in the next few years.
📌 In addition to the difficulty in identifying novel lead compounds suitable for antibacterial drug development, the ability to secure funding for phase-Ill trials and NDA/MAAs, as well as the capacity to generate adequate revenue to get positive net returns on investment for marketed antibacterial drugs, have been major obstacles to antibacterial drug development.
🔍 Recognition of the antibiotic crisis has led to the establishment of targeted funding initiatives for antibiotic development such as the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator
(CARB-X), INCATE, REPAIR Impact Fund, the AMR Action Fund, GARDP, and Access to Medicines testing of new incentives to reimburse pharmaceutical companies such as a subscription ‘Netflix’ model, and legislative initiatives such as the PASTEUR (The Pioneering Antimicrobial Subscriptions To End Up surging Resistance) Act in the United States.
🔴 The need for new antibacterial drugs to treat the increasing global prevalence of drug-resistant bacterial infections has clearly attracted global attention. Along with the encouraging trends in phase-I and -Il antibacterial drug candidates and plans to address issues with the late-stage pipeline, it is imminent to continue to stimulate further antibacterial drug discovery and development.
Link to the article: go.nature.com/43rWlQ2
Published On: /06/2023